��Ŀ����
����Ŀ��һ�������£�CO2��NH3��Ӧ���Ƶ���Ҫ�Ļ�����Ʒ�����谷��3NH3 + 3CO2![]()
 +3H2O��
+3H2O��
��1����̬Nԭ�ӵļ۲�����Ų�ͼΪ____________�������谷�ļ������Ԫ���е縺���ɴ�С��˳��Ϊ____________����Ԫ�ط��ű�ʾ����
��2�������谷�е�ԭ�ӵ��ӻ��������Ϊ____________��
��3�������ϳ������谷�ķ�Ӧ�����д��ڶ������ͻ�ѧ���Ķ������γɣ�����Щ��ѧ���в�����____________����ѡ����ĸ����
a������ b���м� c���Ǽ��Թ��ۼ� d�����Թ��ۼ�
��4�������谷���۵�Ϊ250 �棬���侧��������____________����֪���������۵�Ϊ5.7 �棬���������������۵�����ԭ����____________��
��5�����߿�ѧ�����ķ�չ����ѧ�����Ѻϳ�����̼��������Ԫ���γɵ�ԭ�Ӿ��壮�侧���ṹ��ͼ(a) ��ʾ������������Ϊa pm����NA��ʾ�����ӵ�������ֵ����þ������ܶ���____________ g cm-3��
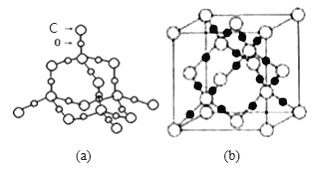
��6��SiO2����ṹƬ����ͼ (b)��ʾ��ͨ�����ǰѲ�1mol ij��ѧ�������յ��������ɸû�ѧ���ļ��ܡ�
��ѧ�� | Si��O | Si��Si | O��O |
����/ KJ��mol��1 | 460 | 176 | 498 |
Si��s)��O2��g)![]() SiO2��s)���÷�Ӧ�ķ�Ӧ�ȡ�H = ___________
SiO2��s)���÷�Ӧ�ķ�Ӧ�ȡ�H = ___________
���𰸡� ![]() O>N>C>H sp2 c ���Ӿ��� �����谷���Ӽ���γ����������������
O>N>C>H sp2 c ���Ӿ��� �����谷���Ӽ���γ���������������� ![]() ��990kJ/mol
��990kJ/mol
��������(1)NΪ7��Ԫ�أ���̬Nԭ�ӵļ۲�����Ų�ͼΪ![]() ��Ԫ�صķǽ�����Խǿ����縺��Խǿ���������谷������Ԫ�طǽ����Թ�ϵΪ��O>N��C��H�����Ը�Ԫ�صĵ縺���ɴ�С��˳���ǣ�O>N��C��H���ʴ�Ϊ��
��Ԫ�صķǽ�����Խǿ����縺��Խǿ���������谷������Ԫ�طǽ����Թ�ϵΪ��O>N��C��H�����Ը�Ԫ�صĵ縺���ɴ�С��˳���ǣ�O>N��C��H���ʴ�Ϊ��![]() ��O>N��C��H��
��O>N��C��H��
(2)���л��������谷�ṹͼ��֪��������Nԭ���γɵĻ�ѧ���У�Nԭ���к���3���۲���Ӷ��ӻ�����Ϊsp2���ʴ�Ϊ��sp2��
(3)���ݷ���ʽ��3NH3 + 3CO2![]()
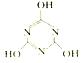 +3H2O���ϳ������谷�ķ�Ӧ�����д��ڶ������ͻ�ѧ���Ķ������γɣ�����Щ��ѧ��ΪN-H�������Թ��ۼ���C=O������û�зǼ��Թ��ۼ��Ķ������γɣ���ѡc��
+3H2O���ϳ������谷�ķ�Ӧ�����д��ڶ������ͻ�ѧ���Ķ������γɣ�����Щ��ѧ��ΪN-H�������Թ��ۼ���C=O������û�зǼ��Թ��ۼ��Ķ������γɣ���ѡc��
(4)�����谷���۵�Ϊ250 �����۵�ϵͣ���̬ʱ���ɷ��Ӿ��壬��֪���������۵�Ϊ5.7 �棬���������������۵�����ԭ��Ϊ�����谷���Ӽ���γ���������������ܣ��ʴ�Ϊ�����Ӿ��壻�����谷���Ӽ���γ���������������ܣ�
(5)���ݾ����ṹͼ����̼��������Ԫ���γɵ�ԭ�Ӿ���Ļ�ѧʽΪCO2�����������ľ����ṹ����(ͼb)��1�������к���Cԭ��8��![]() +6
+6![]() +4=8����Oԭ��16����1mol����������Ϊ44g��8=352g��1mol���������Ϊa3��NApm3���þ������ܶ�=
+4=8����Oԭ��16����1mol����������Ϊ44g��8=352g��1mol���������Ϊa3��NApm3���þ������ܶ�=![]() =
=![]() g cm-3���ʴ�Ϊ��
g cm-3���ʴ�Ϊ��![]() ��
��
(6)1mol�辧���к���2mol Si��Si����1mol�����к���1mol O��O����1mol���������к���4mol Si��O������H=��Ӧ���ܼ���-�������ܼ��ܣ���H=2��176+498-4��460=��990kJ/mol���ʴ�Ϊ����990kJ/mol��

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�����Ŀ����֪��CO(g)��H2O(g)![]() CO2(g)��H2(g)����H����41 kJ��mol��1����ͬ�¶��£��������ͬ�����������ܱ������У�����һ�����ķ�Ӧ�����Ӧ��
CO2(g)��H2(g)����H����41 kJ��mol��1����ͬ�¶��£��������ͬ�����������ܱ������У�����һ�����ķ�Ӧ�����Ӧ��
����������£�
���� ��� | ��ʼʱ�����ʵ����ʵ���/mol | ��ƽ�������ϵ�������仯 | |||
CO | H2O | CO2 | H2O | ||
�� | 1 | 4 | 0 | 0 | �ų�������32.8 kJ |
�� | 0 | 0 | 1 | 4 | �����仯��Q |
����˵���У�����ȷ����(����)
A. �������з�Ӧ��ƽ��ʱ��CO��ת����Ϊ80%
B. ��������CO��ת���ʵ�����������CO2��ת����
C. ��������CO��Ӧ���ʵ���H2O�ķ�Ӧ����
D. ƽ��ʱ����������CO2��Ũ�����
����Ŀ�����з�Ӧ�У����ʾ��ͼ��������
A | B | C | D |
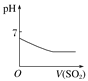
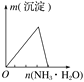
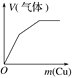
����������ͨ�뵽һ������ˮ�� | ����ˮ���뵽һ�����Ȼ�����Һ�� | ��ͭ�ۼ��뵽һ����Ũ������ | �����ۼ��뵽һ�����Ȼ�����Һ�� |
|
|
|
|
A. A B. B C. C D. D