��Ŀ����
����Ŀ����ѡ������ʵ�鷽���������ʣ������뷽����������ں����ϡ�
A ��ȡ��Һ�� B �ᾧ�� C ��Һ�� D ���� E ���˷� F ������
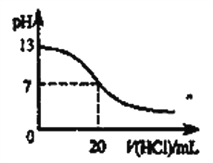
��_____���뱥��ʳ��ˮ��ɳ�ӵĻ���
��_____����ˮ�����͵Ļ���
��_____�������Ȼ�̼���е�Ϊ 76.75�棩�ͼױ����е�Ϊ 110.6�棩�Ļ���
��_____�ӵ��ˮ��Һ����ȡ�⡣
��_____������غ��Ȼ��ƵĻ��Һ�л������ء�
����ͼ��ijͬѧ��Ƶ�����װ��ͼ
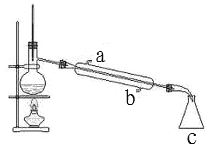
�ٽ�ˮ����_____��a �� b����
��װ�� c ��������_____��
��������ƿ�г������ʯ�����Ƭ����Ŀ����_____��
���𰸡� E C D A B b ��ƿ ��ֹ����
��������������NaCl������ˮ������ɳ���ܣ����ù��˵ķ������룬�ʴ�Ϊ��E��
��ˮ�����ͷֲ㣬���÷�Һ©����Һ���ɣ��ʴ�Ϊ��C��
��CCl4�ͼױ����ܣ����÷е㲻ͬ������룬�ʴ�Ϊ��D��
�ܵⲻ������ˮ���������л��ܼ�������л��ܼ���ȡ������⣬�ʴ�Ϊ��A��
�ݶ��ߵ��ܽ�����¶�Ӱ�첻ͬ����ᾧ���ɷ��������أ��ʴ�Ϊ��B��
�����ٸ�������ԭ��ͨ������ˮ���ʽ�ˮ����b���ʴ�Ϊ��b��
����ͼ��֪��cΪ��ƿ���ʴ�Ϊ����ƿ��
��������ƿ�г������ʯ�����Ƭ����Ŀ���Ƿ�ֹҺ�屩�У��ʴ�Ϊ����ֹ������

����Ŀ��һ�������½�����m g���л�������������ȫȼ�գ�ȼ�պ�ȫ�����ﻺ��ͨ�������������ƣ���ַ�
Ӧ��������ƹ�������n g�������������˵����ȷ����
��� | n | |
A | CH4 | 2m |
B | HCHO | 3m/2 |
C | CH3OH��CH3COOH�Ļ���� | 2m/3 |
D | C2H5OH��CH3COOC2H5�Ļ���� | m |
A. A B. B C. C D. D
����Ŀ��ͨ������������������������ˮ�����ٷ�Һ�ŷŶԻ�������Ⱦ��ͬʱ����K2Cr2O7��ʵ���ҶԺ�����Һ������Cr3+��Fe3+��K+��SO42����NO3��������Cr2O72���������������ù������£�
��֪����Cr(OH)3+OH��=CrO2��+2H2O�� ��2CrO2��+3H2O2+2OH��=2CrO42��+4H2O��M(Cr)=52
��H2O2�����������¾��л�ԭ�ԣ��ܽ�+6��Cr��ԭΪ+3��Cr��
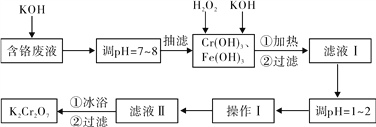
��1�����˹�����Ҫ��ʱ�۲�����ƿ��Һ��߶ȣ�����ﵽ֧�ܿ�λ��ʱӦ���еIJ���Ϊ_____________________________��
��2����Һ���ữǰ�����м��ȵ�Ŀ����____________________________��
��ԡ�����˺�Ӧ��������ˮϴ��K2Cr2O7����Ŀ����________________________��
��3���±���������ʵ��ܽ�����ݣ�
���� | 0�� | 20�� | 40�� | 60�� | 80�� | 100�� |
KCl | 28.0 | 34.2 | 40.1 | 45.8 | 51.3 | 56.3 |
K2SO4 | 7.4 | 11.1 | 14.8 | 18.2 | 21.4 | 24.1 |
K2Cr2O7 | 4.7 | 12.3 | 26.3 | 45.6 | 73.0 | 102.0 |
KNO3 | 13.9 | 31.6 | 61.3 | 106 | 167 | 246.0 |
�����ܽ�����ݣ�����pHѡ����Լ���__________________��
A��ϡ���� B��ϡ���� C��ϡ����
������������������______________________����____________________��
��4����ȡ��Ʒ�ظ��������4.000g���250mL��Һ��ȡ��25.00mL����ƿ�У�����10mL 2 mol��L��1H2SO4�������⻯�ƣ����Ļ�ԭ����ΪCr3+�������ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.2400 mol��L��1Na2S2O3����Һ�ζ���I2+2S2O32��=2I��+S4O62������
�� ��ʵ���й���ȥNa2S2O3����Һ30.00mL�����ò�Ʒ�����ظ���صĴ���Ϊ__________________________����ʽ�����㣬�������������������ʲ����뷴Ӧ����
�� ��װNa2S2O3��Һ�ĵζ����ڵζ�ǰ�����ݵζ���û�����ݣ���õ��ظ���صĴ��Ƚ���______________________���ƫ�ߡ�����ƫ�͡������䡱����
����Ŀ�������ᴿ�ǻ�ѧʵ���е���Ҫ���֣������й��ˡ���������ȡ������ȣ�Ӧ�ù㷺���������м���Ũ������ʵ�������ˮ �� �� �� �� ��ϩ�ij��� ���� �� ʵ�� �Һϳɻ���ϩ�ķ�Ӧ�� ʵ�� װ ����ͼ��
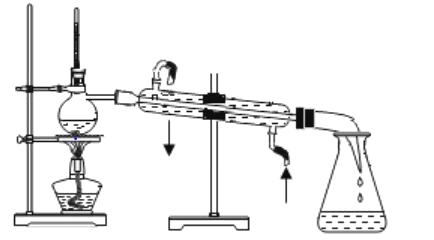
�����õ����й��������£�
��Է������� | �ܶ� / �� gcm�D 3 �� | �е�/�� | �ܽ��� | |
������ | 100 | 0.9618 | 161 | ����ˮ |
�� ��ϩ | 82 | 0.8102 | 83 | ������ˮ |
I.�ϳɷ�Ӧ��
�� a �� ���� 20g ������ �� 2 СƬ���Ƭ����ȴ�������������� 1 ml Ũ �� �� �� b �� ͨ���� ȴˮ��ʼ�������� a �������������¶Ȳ����� 90�森
�� �����ᴿ ��
��Ӧ�ֲ��ﵹ���Һ©�� �� �ֱ������� 5% ̼������Һ��ˮϴ�ӣ� ���� �������ˮ�Ȼ� �ƿ���������һ��ʱ�����ȥ�Ȼ��ƣ�����ͨ�� ���� �õ����� �� ��ϩ 10g �� �ش��������⣺
��1���������Ƭ�������� _____�� �� �� �� �� һ �� ʱ �� �� �� �� �� �� �� �� Ƭ �� Ӧ �� �� ȡ ����ȷ������ _____���� ��ĸ���� ����
A ���������� B ����ȴ��
C �����貹�� D ����������
��2���ڱ� ʵ����� ������ ������Ӧ�ôӷ�Һ©���� _______���� ���Ͽڵ��� ���� ���¿ڵ� �� ������ ������ _____��
��3�������ᴿ�� �� �� ������ˮ�Ȼ��Ƶ�Ŀ���� _____��
��4���� �� ��ϩ�ֲ��� ���� ���� �� ���������õ��������� ________���� ��ĸ���� ����
A �� ���� ��ƿ B ���¶ȼ� C ����Һ©��
D ��ţ�ǹ� E ����ƿ��