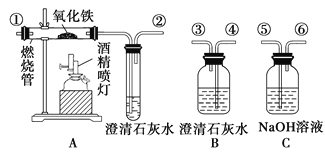
ЬтФПФкШн
ЁОЬтФПЁПЭЈЙ§ГСЕэЃбѕЛЏЗЈДІРэКЌИѕЗЯЫЎЃЌМѕЩйЗЯвКХХЗХЖдЛЗОГЕФЮлШОЃЌЭЌЪБЛиЪеK2Cr2O7ЁЃЪЕбщЪвЖдКЌИѕЗЯвКЃЈКЌгаCr3+ЁЂFe3+ЁЂK+ЁЂSO42ЃЁЂNO3ЃКЭЩйСПCr2O72ЃЃЉЛиЪегыдйРћгУЙЄвеШчЯТЃК
вбжЊЃКЂйCr(OH)3+OHЃ=CrO2Ѓ+2H2OЃЛ Ђк2CrO2Ѓ+3H2O2+2OHЃ=2CrO42Ѓ+4H2OЃЛM(Cr)=52
ЂлH2O2дкЫсадЬѕМўЯТОпгаЛЙдадЃЌФмНЋ+6МлCrЛЙдЮЊ+3МлCrЁЃ

ЃЈ1ЃЉГщТЫЙ§ГЬжавЊМАЪБЙлВьЮќТЫЦПФквКУцИпЖШЃЌЕБПьДяЕНжЇЙмПкЮЛжУЪБгІНјааЕФВйзїЮЊ_____________________________ЁЃ
ЃЈ2ЃЉТЫвКЂёЫсЛЏЧАЃЌНјааМгШШЕФФПЕФЪЧ____________________________ЃЛ
БљдЁЁЂЙ§ТЫКѓЃЌгІгУЩйСПРфЫЎЯДЕгK2Cr2O7ЃЌЦфФПЕФЪЧ________________________ЁЃ
ЃЈ3ЃЉЯТБэЪЧЯрЙиЮяжЪЕФШмНтЖШЪ§ОнЃК
ЮяжЪ | 0Ёц | 20Ёц | 40Ёц | 60Ёц | 80Ёц | 100Ёц |
KCl | 28.0 | 34.2 | 40.1 | 45.8 | 51.3 | 56.3 |
K2SO4 | 7.4 | 11.1 | 14.8 | 18.2 | 21.4 | 24.1 |
K2Cr2O7 | 4.7 | 12.3 | 26.3 | 45.6 | 73.0 | 102.0 |
KNO3 | 13.9 | 31.6 | 61.3 | 106 | 167 | 246.0 |
ИљОнШмНтЖШЪ§ОнЃЌЕїНкpHбЁдёЕФЪдМСЪЧ__________________ЁЃ
AЃЎЯЁбЮЫс BЃЎЯЁСђЫс CЃЎЯЁЯѕЫс
ВйзїЂёОпЬхВйзїВНжшЮЊЂй______________________ЁЂЂк____________________ЁЃ
ЃЈ4ЃЉГЦШЁВњЦЗжиИѕЫсМиЪдбљ4.000gХфГЩ250mLШмвКЃЌШЁГі25.00mLгкзЖаЮЦПжаЃЌМгШы10mL 2 molЁЄLЃ1H2SO4КЭзуСПЕтЛЏФЦЃЈИѕЕФЛЙдВњЮяЮЊCr3+ЃЉЃЌЗХгкАЕДІ5minЃЌШЛКѓМгШы100mLЫЎЃЌМгШы3mLЕэЗлжИЪОМСЃЌгУ0.2400 molЁЄLЃ1Na2S2O3БъзМШмвКЕЮЖЈЃЈI2+2S2O32Ѓ=2IЃ+S4O62ЃЃЉЁЃ
Ђй ШєЪЕбщжаЙВгУШЅNa2S2O3БъзМШмвК30.00mLЃЌЫљЕУВњЦЗЕФжажиИѕЫсМиЕФДПЖШЮЊ__________________________ЃЈСаЪНВЛМЦЫуЃЌЩшећИіЙ§ГЬжаЦфЫќдгжЪВЛВЮгыЗДгІЃЉЁЃ
Ђк ШєзАNa2S2O3БъзМвКЕФЕЮЖЈЙмдкЕЮЖЈЧАгаЦјХнЕЮЖЈКѓУЛгаЦјХнЃЌВтЕУЕФжиИѕЫсМиЕФДПЖШНЋЃК______________________ЃЈЬюЁАЦЋИпЁБЁЂЁАЦЋЕЭЁБЁЂЛђЁАВЛБфЁБЃЉЁЃ
ЁОД№АИЁП АЮЕєЮќТЫЦПЩЯЕФЯ№ЦЄЙмЃЌДгЮќТЫЦПЩЯПкЕЙГіШмвКГ§ШЅH2O2 Г§ШЅОЇЬхБэУцЕФдгжЪЃЌВЂМѕЩйОЇЬхЕФЫ№ЪЇ B еєЗЂЃЈХЈЫѕЃЉ НсОЇ ГУШШЙ§ТЫ ![]() ЁС100% ЦЋИп
ЁС100% ЦЋИп
ЁОНтЮіЁПЪЕбщЪвЖдКЌИѕЗЯвК(КЌгаCr3+ЁЂFe3+ЁЂK+ЁЂSO42-ЁЂNO3-КЭЩйСПCr2O72-)ЛиЪеЃЌЯШМгKOHАбCr3+ЁЂFe3+зЊЛЏГСЕэЗжРыГіРДЃЌдйЯђГСЕэжаМгЫЋбѕЫЎКЭKOHЃЌАбCr(OH)3зЊЛЏЮЊCrO42-ЃЌЫсадЬѕМўЯТCrO42-зЊЛЏЮЊCr2O72-ЃЌЭЈЙ§еєЗЂХЈЫѕЃЌЙ§ТЫЕУЕНK2Cr2O7ЁЃ
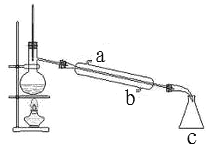
(1)ЪЕбщЪБЃЌЕБЮќТЫЦПжавКУцИпЖШПьДяЕНжЇЙмПкЮЛжУЪБЃЌЮЊЗРжЙвКЬхНјШыЦфЫќзАжУгІИУЕЙГіВПЗжвКЬхЃЌЫљвдЦфВйзїЗНЗЈЪЧЃКАЮЕєЮќТЫЦПЩЯЕФЯ№ЦЄЙмЃЌДгЮќТЫЦПЩЯПкЕЙГіШмвКЃЛЙЪД№АИЮЊЃКАЮЕєЮќТЫЦПЩЯЕФЯ№ЦЄЙмЃЌДгЮќТЫЦПЩЯПкЕЙГіШмвКЃЛ
(2)H2O2ВЛЮШЖЈЃЌЪмШШвзЗжНтЃЌЫљвдЭЈЙ§МгШШРДГ§ШЅH2O2ЃЛK2Cr2O7дкРфЫЎжаЕФШмНтЖШНЯаЁЃЌгУЩйСПРфЫЎЯДЕгK2Cr2O7ЃЌФмГ§ШЅОЇЬхБэУцВаСєЕФдгжЪЃЌЛЙФмМѕаЁK2Cr2O7ЕФЫ№КФЃЌЙЪД№АИЮЊЃКГ§ШЅH2O2ЃЛГ§ШЅОЇЬхБэУцВаСєЕФдгжЪЃЌМѕаЁK2Cr2O7ЕФЫ№КФЃЛ
(3)K2Cr2O7ОпгаЧПбѕЛЏадЃЌФмЙЛбѕЛЏбЮЫсЃЌШєМгШыЯѕЫсЃЌЯѕЫсМиЕФШмНтЖШНЯДѓЃЌВЛШнвзгыK2Cr2O7ЗжРыЃЌЙЪбЁЯЁСђЫсЫсЛЏЃЌЙЪбЁBЃЛИљОнБэжаЪ§ОнПЩжЊЮТЖШНЯИпЪБK2Cr2O7ЕФШмНтЖШНЯДѓЃЌЦфЫќЮяжЪЕФШмНтЖШНЯаЁЃЌеєЗЂХЈЫѕЪЙдгжЪзЊЛЏЮЊЙЬЬхЮіГіЃЌЮТЖШНЯИпЪБK2Cr2O7ВЛЮіГіЙЬЬхЃЌЫљвдвЊГУШШЙ§ТЫЃЛЙЪД№АИЮЊЃКBЃЛеєЗЂНсОЇЃЛГУШШЙ§ТЫЃЛ
(4)ЂйгЩЗДгІCr2O72-+6I-+14H+=2Cr3++3I2+7H2OЃЛI2+2S2O32-=2I-+S4O62-ПЩЕУЗДгІЕФЙиЯЕЪНЮЊCr2O72-ЁЋ3I2ЁЋ6S2O32-ЃЌИљОнЙиЯЕЪНМЦЫуЃЌ
Cr2O72-ЁЋ3I2ЁЋ6S2O32-
1mol 6mol
n 0.2400ЁС30ЁС10-3mol
дђ250mlКЌжиИѕЫсМиЕФЮяжЪЕФСПЮЊn=![]() ЁС10ЃЌдђЫљЕУВњЦЗжажиИѕЫсМиДПЖШЮЊ
ЁС10ЃЌдђЫљЕУВњЦЗжажиИѕЫсМиДПЖШЮЊ![]() ЁС100%ЃЌЙЪД№АИЮЊЃК
ЁС100%ЃЌЙЪД№АИЮЊЃК ![]() ЁС100%ЃЛ
ЁС100%ЃЛ
ЂкзАNa2S2O3БъзМвКЕФЕЮЖЈЙмдкЕЮЖЈЧАгаЦјХнЕЮЖЈКѓУЛгаЦјХнЃЌЛсдьГЩV(БъзМ)ЦЋДѓЃЌдђМЦЫуГіNa2S2O3ЕФЮяжЪЕФСПЦЋДѓЃЌжиИѕЫсМиЕФЮяжЪЕФСПЦЋДѓЃЌдђВтЕУЕФжиИѕЫсМиЕФДПЖШНЋЦЋИпЃЛЙЪД№АИЮЊЃКЦЋИпЁЃ

 ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ
ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИЁОЬтФПЁПЖдгквзШМЁЂвзБЌЁЂгаЖОЕФ ЛЏбЇЮяжЪЃЌЭљЭљЛсдкЦфАќзАЩЯЬљЩЯЮЃЯеОЏИцБъЧЉЃЎЯТУцЫљ СаЕФЮяжЪжаЃЌБъЧЉЬљДэСЫЕФЪЧ
бЁЯю | A | B | C | D |
ЮяжЪЕФЛЏбЇЪН | ЧтбѕЛЏФЦ | Н№ЪєЙЏ | ЫФТШЛЏЬМ | бЬЛЈБЌжё |
ЮЃЯеОЏИцБъЧЉ |
|
|
|
|
A. A B. B C. C D. D
ЁОЬтФПЁПЬњМАЦфЛЏКЯЮядкЙЄХЉвЕЩњВњжагаживЊЕФзїгУЁЃ
ЃЈ1ЃЉвбжЊЃКЂйC(s)+O2(g)=CO2(g) ЁїH1=-393.5kJ/molЃЛ
ЂкC(s)+CO2(g)=2CO(g) ЁїH2=+172.5kJ/mol
Ђл4Fe(s)+3O2(g)=2Fe2O3(s) ЁїH3=-1651.0kJ/mol
COЛЙдFe2O3ЕФШШЛЏбЇЗНГЬЪНЮЊ__________________________________________ЁЃ
ЃЈ2ЃЉИпТЏСЖЬњВњЩњЕФИпТЏЦјжаКЌгаCOЁЂH2ЁЂCO2ЕШЦјЬхЃЌгУCOКЭH2дкДпЛЏМСзїгУЯТКЯГЩМзДМЃЌЪЧМѕЩйЮлШОЁЂНкдМФмдДЕФаТОйДыЃЌЗДгІдРэЃКCO(g)+2H2(g)![]() CH3OH(g) ЁїHЁЃдкЬхЛ§ВЛЭЌЕФСНИіКуШнУмБеШнЦїжаЗжБ№ГфШы1molCOКЭ2mol H2ЃЌВтЕУЦНКтЛьКЯЮяжаCH3OHЕФЬхЛ§ЗжЪ§дкВЛЭЌбЙЧПЯТЫцЮТЖШЕФБфЛЏШчЭМЁЃ
CH3OH(g) ЁїHЁЃдкЬхЛ§ВЛЭЌЕФСНИіКуШнУмБеШнЦїжаЗжБ№ГфШы1molCOКЭ2mol H2ЃЌВтЕУЦНКтЛьКЯЮяжаCH3OHЕФЬхЛ§ЗжЪ§дкВЛЭЌбЙЧПЯТЫцЮТЖШЕФБфЛЏШчЭМЁЃ
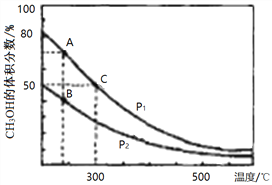
ЂйдкЩЯЭМAЁЂBЁЂCШ§ЕужаЃЌбЁГіЖдгІЯТБэЮяРэСПзюаЁЕФЕуЁЃ
ЗДгІЫйТЪ | ЦНКтГЃЪ§K | ЦНКтзЊЛЏТЪІС |
_________ | _________ | _________ |
Ђкдк300ЁцЪБЃЌЯђCЕуЦНКтЬхЯЕжадйГфШы0. 5molCOЁЂ1.0molH2КЭ0.5molЕФCH3OHЃЌИУЗДгІЯђ_________ЗНЯђНјааЃЈЬюЁАе§ЗДгІЁБЁЂЁЎФцЗДгІЁБЛђЁАВЛвЦЖЏЁБЃЉЁЃ
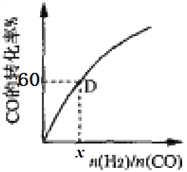
ЂлвЛЖЈЮТЖШЯТЃЌCOЕФзЊЛЏТЪгыЦ№ЪМЭЖСЯБШ[n(H2)/n(CO)]ЕФБфЛЏЙиЯЕЭМЫљЪОЃЌВтЕУDЕуЧтЦјЕФзЊЛЏТЪЮЊ40%ЃЌдђx=_____________ЁЃ
ЃЈ3ЃЉШ§ТШЛЏЬњЪЧвЛжжживЊЕФЛЏКЯЮяЃЌПЩвдгУРДИЏЪДЕчТЗАхЁЃФГИЏЪДЗЯвКжаКЌга0.5molЁЄL-1Fe3+КЭ0.26molЁЄL-1ЕФCu2+ЃЌгћЪЙFe3+ЭъШЋГСЕэ[c(Fe3+)Ём4ЁСl0-5]ЖјCu2+ВЛГСЕэЃЌдђашПижЦШмвКpHЕФЗЖЮЇЮЊ_________ЁЃ[KspCu(OH)2=2.6ЁСl0-19ЃЛKspFe(OH)3=4ЁСl0-38]
ЃЈ4ЃЉФЊЖћбЮЃЌМДСљЫЎКЯСђЫсбЧЬњяЇОЇЬхЃЌЪЧвЛжжживЊЕФЛЏЙЄдСЯЃЌдкПеЦјжаЛКТ§ЗчЛЏМАбѕЛЏЃЌгћжЄУївЛЦПОУжУЕФФЊЖћбЮвбОВПЗжбѕЛЏЃЌашвЊНјааЪЕбщВйзїЪЧЃКШЁЩйСПбљЦЗЃЌМгЮобѕЫЎШмНтЃЌНЋШмвКЗжГЩСНЗнЃЌ______________________________________ЃЌдђжЄУїИУбљЦЗвбВПЗжбѕЛЏЁЃ