��Ŀ����
����Ŀ�����������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ����
A����2H2(g)��O2(g)=2H2O(g)��H����483.6kJ��mol��1����H2ȼ����Ϊ241.8kJ��mol��1
B����C(ʯī��s)=C(���ʯ��s)��H>0����ʯī�Ƚ��ʯ�ȶ�
C����֪NaOH(aq)��HCl(aq)=NaCl(aq)��H2O(l)��H����57.4kJ��mol��1����20.0gNaOH������ϡ������ȫ�кͣ��ų�28.7kJ������
D����֪2C(s)��2O2(g)=2CO2(g)��H1��2C(s)��O2(g)=2CO(g)��H2������H1>��H2
���𰸡�B
��������
���������A��ѡ�������ɵ�ˮ�����壬Ӧ����Һ̬ˮ�����Բ��ܵó�������ȼ������241.8kJ/mol����A����B����֪C(ʯī��s)=C(���ʯ��s)��H��0����Ӧ�����ȷ�Ӧ��ʯī�������ڽ��ʯ������Խ��Խ�ȶ�������ʯī�Ƚ��ʯ�ȶ�����B��ȷ��C����֪NaOH(ag)+HCl(aq)=NaCl(aq)+H2O(l)��H=-57.4kJ/mol����20.0gNaOH���ʵ���Ϊ0.5mol��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.7kJ����������C����D����֪C(s)+O2(g)=CO2(g)��H1����C(s)+![]() O2(g)=CO(g)��H2������-���õ���CO(g)+
O2(g)=CO(g)��H2������-���õ���CO(g)+![]() O2(g)=CO2(g)��H1-��H2��һ����̼ȼ�����ɶ�����̼�Ƿ��ȷ�Ӧ���ʱ��Ǹ�ֵ��������H1����H2����D����ѡB��
O2(g)=CO2(g)��H1-��H2��һ����̼ȼ�����ɶ�����̼�Ƿ��ȷ�Ӧ���ʱ��Ǹ�ֵ��������H1����H2����D����ѡB��

����Ŀ��������KMnO4��H2C2O4�����ᣩ��Ӧ�о�Ӱ�췴Ӧ���ʵ����ء�һʵ��С����ͨ���ⶨ��λʱ��������CO2�����ʣ�̽��ij��Ӱ�컯ѧ��Ӧ���ʵ����أ����ʵ�鷽�����£�KMnO4��Һ���ữ����

ʵ����� | A��Һ | B��Һ |
�� | 20 mL 0.1 mol��L��1H2C2O4��Һ | 30 mL 0.01 mol��L��1KMnO4��Һ |
�� | 20 mL 0.2 mol��L��1H2C2O4��Һ | 30 mL 0.01 mol��L��1KMnO4��Һ |
��1���÷�Ӧ�����ӷ���ʽ ������֪H2C2O4�Ƕ�Ԫ������
��2����ʵ��̽������ ���ضԻ�ѧ��Ӧ���ʵ�Ӱ�졣��ͬʱ������Ͳ������CO2�������С��ϵ�� �� ����ʵ���������
��3����ʵ������2 minĩ�ռ���2.24 mL CO2����״���£�������2 minĩ�� c(MnO4��)��__________mol/L��������Һ���Ϊ50 mL��
��4����ͨ���ⶨһ��ʱ����CO2��������ȽϷ�Ӧ���ʣ���ʵ�黹��ͨ���ⶨ ���Ƚϻ�ѧ��Ӧ���ʡ���һ�����ɣ�
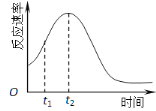 ��5��С��ͬѧ���ַ�Ӧ������������ͼ������t1��t2ʱ�������ʱ�����Ҫԭ������ǣ�
��5��С��ͬѧ���ַ�Ӧ������������ͼ������t1��t2ʱ�������ʱ�����Ҫԭ������ǣ�
�� ���� ��
