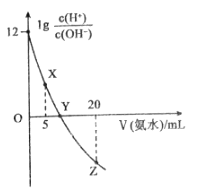
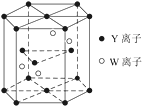
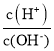��Ŀ����
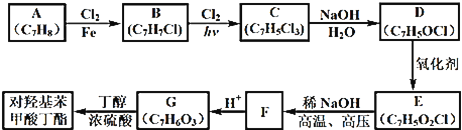
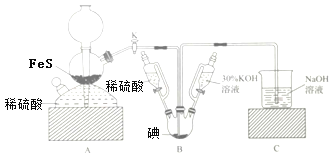
����Ŀ����KIO3��H2S��Ӧ�Ʊ�KI���壬װ����ͼ��ʾ��ʵ�鲽�裺�ټ��װ�õ������ԣ��ڹر�K���ڸ�װ���м�����Ӧ�Լ�����װ��B�е���30%��KOH��Һ���Ƶ�KIO3���۴�K��ͨ��H2Sֱ�����Ϳ��Ƶ�KI��ͬʱ�л�ɫ�������ɣ��ܹر�K����������Һ����ϡ���ᣬˮԡ���ȣ�����ݳ�H2S���ݰ�װ��B�л��Һ�����ձ���Ϊ��ȥ���ᣬ��������̼�ᱵ���پ���һϵ�в����ɵó�Ʒ�������йظ�ʵ�������������ǣ� ��
A.����ڿɹ۲쵽�����ܽ⣬��Һ���ػ�ɫ��Ϊ��ɫ
B.�����װ��B�з�����Ӧ�����ӷ���ʽΪ3H2S+IO3-�T3S��+3H2O+I-
C.װ��C������������Һ����������������
D.�������һϵ�в���Ϊ���ˣ�ϴ�ӣ��ϲ���Һ��ϴҺ������
���𰸡�D
��������
����װ�ã�Aװ����FeS��ϡ�����Ʊ�H2S��Bװ���ɵ���KOH�Ʊ�KIO3����ӦΪ��3I2+6KOH�TKIO3+5KI+3H2O����H2Sͨ��B����KIO3��Һ������Ӧ��3H2S+IO3-�T3S��+3H2O+I-����������Һ����ϡ���ᣬˮԡ���ȣ�����ݳ�H2S��Cװ�����ն����H2S����װ��B�л��Һ�����ձ���Ϊ��ȥ���ᣬ��������̼�ᱵ���õ����ᱵ���������ˣ�ϴ�ӣ��ϲ���Һ��ϴҺ�������ᾧ�õ�KI���壬�ݴ˷������
A��������е���30%����������Һ������Ӧ3I2+6KOH�TKIO3+5KI+3H2O���ػ�ɫ(��ˮ��ɫ)��Һ�����ɫ����A��ȷ��
B���������װ��B���ɻ�ɫ������ΪS����ӦΪ3H2S+IO3-�T3S��+3H2O+I-����B��ȷ��
C��β��ֻ����H2S����Ⱦ����������ֱ���ŷţ�װ��C������������Һ���������������⣬��C��ȷ��
D��������а�װ��B�л��Һ�����ձ���Ϊ��ȥ���ᣬ��������̼�ᱵ���õ����ᱵ���������ˣ�ϴ�ӣ��ϲ���Һ��ϴҺ�������ᾧ�õ���Ʒ����������D����
��ѡD��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�����Ŀ�����к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȣ��Ը���ʵ��ش��������⣺
��1��ȷ����8.2g�������������������ʵ���Ʒ�����500mL������Һ������ʱ����Ʒ�ɷ���___(����ĸ)������
A.С�ձ��� B.�ྻֽƬ�� C.������
��2���ζ�ʱ����0.2000mol��L-1���������ζ�������Һ������ѡ��__(����ĸ)��ָʾ����
A.���� B.ʯ�� C.��̪
��3���ζ������У��۾�Ӧע��___��
��4�������±����ݣ����㱻���ռ���Һ�����ʵ���Ũ����__mol��L-1���ռ���Ʒ�Ĵ�����___(������λ����)��
�ζ����� | ������Һ���(mL) | ������� | |
�ζ�ǰ�Ŀ̶�(mL) | �ζ���Ŀ̶�(mL) | ||
��һ�� | 10.00 | 0.40 | 20.50 |
�ڶ��� | 10.00 | 4.10 | 24.00 |
��5������ʵ�������Եζ��������ʲô�����(�ƫ�ߡ���ƫ�͡�����Ӱ�족)
�ٹ۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ����___��
��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ����ζ����___��
����Ŀ��������ᣨHN3����Ī����[��NH4��2SO4��FeSO4��6H2O]�����ֳ���ԭ�ϡ�
��1���������������ˮ��25��ʱ������ĵ��볣��ΪKa=10��10-5��
�����������ˮ��Һ�еĵ��뷽��ʽΪ_______
��0.2mol/L��HN3��Һ��0.1mol/L��NaOH��Һ�������Ϻָ���25�棬��ʱ����Һ�����ԣ�������Һ�и����Ӻ�HN3����Ũ���ɴ�С��˳��Ϊ_______��
��2����FeSO4��Һ�У����루NH4��2SO4������Ʊ�Ī���ξ���[��NH4��2Fe��SO4��2��6H2O]��Ϊ�˲ⶨ��Ʒ���ȣ���ȡag��Ʒ����ˮ�����Ƴ�500mL��Һ����Ũ��Ϊcmol/L�����Ը��������Һ�ζ���ÿ����ȡ����Һ�����Ϊ25.00mL��ʵ������¼���£�����֪Ī���εķ�����Ϊ392��
ʵ����� | ��һ�� | �ڶ��� | ������ |
����KMnO4��Һ���/mL | 25.52 | 25.02 | 24.98 |
������Ī������Һ����ʹ�õIJ������������ձ��Ͳ��������_______
�ڵζ��յ��������_______��ͨ��ʵ�����ݣ�����ò�Ʒ�Ĵ���Ϊ_______���ú���ĸa��c��ʽ�ӱ�ʾ����
���ϱ���һ��ʵ���м�¼�������Դ��ں����Σ���ԭ�������_______��
A ��һ�εζ�ʱ����ƿ�ô�װҺ��ϴ
B �����Ը�����ر�Һ����ʱ����������ֱ���
C �ζ�ǰ��ʽ�ζ����м��촦�����ݣ��ζ�������������ʧ