��Ŀ����
12��ij�����о���ѧϰС�����ô��Σ������������ʰ�������ɳ��CaCl2��MgCl2��Na2SO4�����ᴿ������0.4mol/L 450mL NaCl��Һ��������Ʋ�ʵʩ������ʵ�飬�������ѧ����֪ʶ�ش��������⣺��1��ȡһ�����Ĵ��������ձ��м�ˮ�ܽ⣬���ӵ�ˮ��ӦΪ��B������ѡ�
A�����ˮ��ɽ�ϡ����Һ B������������ˮ�����ܽ�Ĺ����ܽ⼴ֹ
��2������1�������ƵĴ�������Һ���˺�ȡ��Һ��������ij����Լ�����������Լ��ɴ�����������Һ��ѡ�� ��Na2CO3��Һ ��KOH��Һ ��AgNO3��Һ ��NaOH��Һ ��NaHCO3��Һ ��BaCl2��Һ������ȷ���Լ��ͼ����˳��ӦΪCE������ѡ���ѡ����ѡ����ѡ�����÷֣�
A���٢ڢ�B���ڢޢ�C���ܢޢ�D���٢ܢ�E���ޢ٢�F���ޢڢ�
�����õ�����Һ�����˺�ȡ��Һ�������������ᣬ��ַ�Ӧ��Ի����Һ�������������ˡ�ϴ�ӡ�����������õ�������NaCl���壬���������������������в������������ǽ��裬ʹ��Һ���Ⱦ��ȣ�
��3�����ã�2�������õ�NaCl�����������������Һ����������ƽ�ϳ���11.7g NaCl���壮���ƹ�������Ҫʹ�õIJ��������У��ձ�����������500mL����ƿ�ͽ�ͷ�ιܣ�
��4�����ƽ�����ͬѧ�Ƕ�ʵ���г��ֵ�ijЩ������������������Ƶ���ҺŨ�ȵ�Ӱ������˷����������д�������ᵼ������������Һ��NaCl��Ũ�����0.4mol/L��ƫ���ǣ����ƫ����ƫС��������Ӱ�족��
����������2������û��ʹ�����ᴦ����Һ�������ƫС��
�ڶ���ʱ���Ӱ�Һ�棬�����ƫ��
�����µߵ�ҡ�Ⱥ�Һ����ڿ̶��ߣ�δ��ʱ��ˮ���̶��ߣ��������Ӱ�죮
���� ��1���ܽ����ʱ�������ˮ��ʹ����ǡ���ܽ⼴�ɣ�
��2�����ݺ��е�������CaCl2��MgCl2��Na2SO4�����ݼ��ܳ�ȥ���ʻ��������������ʵ�ԭ��ѡ������ʵij����Լ��ǣ���Na2CO3��Һ ��NaOH��Һ ��BaCl2��Һ��Ȼ����ݢ�Na2CO3��Һ�������Dz���Ҫ��ȥCaCl2����Ҫ��ȥ������BaCl2���ʢ�Na2CO3��Һ�ļ���һ��Ҫ�ڢ�BaCl2��Һ֮�ó������Լ���˳��
��������������Ϊ��ֹ��Һ��ֲ�������ɷɽ�����Ҫ�ò��������裬ʹ��Һ���Ⱦ��ȣ�
��3������������Һ���ѡ���������ƿ������m=CVM������Ҫ���ʵ��������������Ʋ���ѡ����Ҫ��������
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1���ܽ����ʱ������������ˮ��ʹ����ǡ���ܽ⼴�ɣ�
��ѡB��
��2����ȥʳ���е�CaCl2�â�Na2CO3��Һ����ȥMgCl2�â�NaOH��Һ����ȥNa2SO4�â�BaCl2��Һ������Na2CO3��Һ�������Dz���Ҫ��ȥCaCl2����Ҫ��ȥ������BaCl2���ʢ�Na2CO3��Һ�ļ���һ��Ҫ�ڢ�BaCl2��Һ֮����ȷ���Լ��ͼ����˳��ӦΪ���ޢ٢ܻ�ޢܢٻ�ܢޢ٣�
��������������Ϊ��ֹ��Һ��ֲ�������ɷɽ�����Ҫ�ò��������裬ʹ��Һ���Ⱦ��ȣ�
��ѡCE�����裬ʹ��Һ���Ⱦ��ȣ�
��3������0.4mol/L 450mL NaCl��Һ��Ӧѡ��500mL����ƿ��ʵ������500mL��Һ����Ҫ�����Ȼ��Ƶ�����m=0.4mol/L��58.5g/mol��0.5L=11.7g��
����һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���õ�������Ϊ����ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ����Ի���Ҫ������Ϊ����ͷ�ιܣ� 500mL����ƿ��
�ʴ�Ϊ��11.7�� ��ͷ�ιܣ� 500mL����ƿ��
��4������������2������û��ʹ�����ᴦ����Һ����õ����Ȼ����к������ʣ����³�ȡ���Ȼ�������ƫС�����ʵ����ʵ���ƫС����ҺŨ��ƫС��
�ʴ�Ϊ��ƫС��
�ڶ���ʱ���Ӱ�Һ�棬������Һ���ƫС����ҺŨ��ƫ��
�ʴ�Ϊ��ƫ��
�����µߵ�ҡ�Ⱥ�Һ����ڿ̶��ߣ�δ��ʱ��ˮ���̶��ߣ�������ȷ����ҺŨ��ȷ��Ӱ�죻
�ʴ�Ϊ����Ӱ�죮
���� ���⿼���˴��ε��ᴿ��һ�����ʵ���Ũ����Һ�������Լ����������ѶȲ�����ȷ�����к����������ӵ����ʣ�ѡ����ʵij��Ӽ�������˳���ǽ���ؼ���ע��ʵ��Ļ�������������ע�����

| A�� | ʹ����-��������ȼ�ϵ����Ϊ��⾫��ͭ�ĵ�Դ�������������仯128 g������-��������ȼ�ϵ�����������ı�״���¿��������Ϊ112 L������������������������Ϊ20%�� | |
| B�� | ���ڵ��ˮʱ�������������ƣ�����ˮ��������Һ��pH���ᱣ�ֲ��� | |
| C�� | �������ͭ��ҺһС��ʱ���Ϊ��ʹ�������Һ��ԭ�����Լ���������Cu��OH��2 | |
| D�� | �������ͭ��Һ��������1.12 L����״����ʱ��ת�Ƶ���0.2 mol |
| A�� | һ�������£�����Ӧ�������ӿ컯ѧ��Ӧ���� | |
| B�� | �ܹ�������Ч��ײ�ķ��ӽл���� | |
| C�� | ����Ӽ�����������ײΪ��Ч��ײ | |
| D�� | ����ѹǿ���϶���ӿ컯ѧ��Ӧ���� |
| A�� | ����£�ȼ��1mol S�ų�������Ϊ297.23 kJ | |
| B�� | S��g��+O2��g���TSO2��g���ų�����������297.23 kJ | |
| C�� | S��g��+O2��g���TSO2��g���ų�������С��297.23 kJ | |
| D�� | �γ�1molSO2�Ļ�ѧ�����ͷŵ����������ڶ���1molS��s����1molO2��g���Ļ�ѧ�������յ������� |
| A�� | K��L�γɵ����ֻ����������������ӵĸ����Ⱦ�Ϊ1��2���Ҿ�����ˮ��Ӧ | |
| B�� | ���ݷǽ�����ǿ������������Z������������Ƴ�W����������� | |
| C�� | L�ļ��⻯��ķе�����ȶ��Ծ�����R�ļ��⻯�� | |
| D�� | ������BaCl2��Һ�зֱ�ͨ��RL2��WL2�����ް�ɫ�������ɣ���һ��ʱ���ͨ��RL2��һ���п��ܲ������� |
| A�� | �����������ӵĵ���ʽ�ǣ� | |
| B�� | •������̼���ӵı���ģ���� | |
| C�� | �������������ͨʽ��CnH2n-6��n��6�� | |
| D�� | ��12C��14C��ԭ�ӽṹʾ��ͼ���ɱ�ʾΪ  |
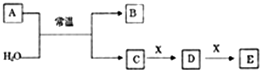 A��B��C��D��E��X����ѧ���������������ͼ��ʾת����ϵ������������ͷ�Ӧ������ȥ������֪A�ɶ����ڷǽ���Ԫ����ɣ�B����Ư�����ҹ����ֽ⣮
A��B��C��D��E��X����ѧ���������������ͼ��ʾת����ϵ������������ͷ�Ӧ������ȥ������֪A�ɶ����ڷǽ���Ԫ����ɣ�B����Ư�����ҹ����ֽ⣮ H++Cl-+HClO��
H++Cl-+HClO��