��Ŀ����
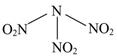
��6�֣�X��Y��Z��RΪǰ������Ԫ�أ���ԭ��������������XY2�Ǻ���ɫ���壻X����Ԫ�ؿ��γ�XH3��Z��̬ԭ�ӵ�M����K���������ȣ�R2+���ӵ�3d�������9�����ӡ���ش��������⣺
��1��Y��̬ԭ�ӵĵ����Ų�ʽ�� ��
��2��R2+��ˮ�������У��ṩ�µ��ӶԵ�ԭ���� ��
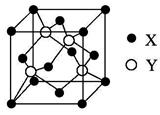
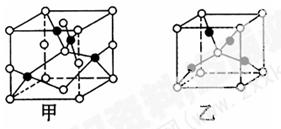
��3��Z��ijԪ���γɵĻ�����ľ�������ͼ��ʾ���������������������ӵĸ������� ��
��4����R���ʵķ�ĩ����XH3��Ũ��Һ�У�ͨ��Y2����ַ�Ӧ����Һ������ɫ���÷�Ӧ�����ӷ���ʽ�� ��

��1��Y��̬ԭ�ӵĵ����Ų�ʽ�� ��
��2��R2+��ˮ�������У��ṩ�µ��ӶԵ�ԭ���� ��
��3��Z��ijԪ���γɵĻ�����ľ�������ͼ��ʾ���������������������ӵĸ������� ��
��4����R���ʵķ�ĩ����XH3��Ũ��Һ�У�ͨ��Y2����ַ�Ӧ����Һ������ɫ���÷�Ӧ�����ӷ���ʽ�� ��
��1��1s22s22p4 ;��2��O; ��3��2:1 ��4��2Cu+8NH3��H2O+O2=2[Cu(NH3)4]2++4OH-+6H2O
��������������������֪��X��N��Y��O��Z��Mg��R��Cu����1��O��̬ԭ�ӵĵ����Ų�ʽ��1s22s22p4 ; ��2��Cu2+��ˮ�������У��ṩ�µ��ӶԵ�ԭ����H2O�����е�Oԭ�ӣ���3�����ݾ����ṹʾ��ͼ��֪�������ӣ�1+8��1/8=2�������ӣ�4��1/2+2=4�����Ծ������������������ӵĸ�������4��2 = 2��1����40�������⣬��ϵ����غ㡢ԭ���غ�ɵ���Ӧ��Ӧ�����ӷ���ʽ��2Cu+8NH3��H2O+O2=2[Cu(NH3)4]2++4OH-+6H2O��

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
�����Ŀ
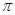 ����___________mol��
����___________mol��