��Ŀ����
��ͼ���ֽ�����������������������ͼ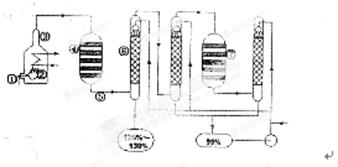
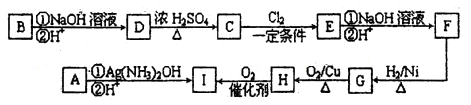
��1���ڢܴ������������������ܵ��豸������ ���ô�������Ӧ�ķ���ʽΪ ��Ϊ�����������IJ��ʣ��ô�Ӧ���� ������¹��̡����ȹ��̡���Ϊ�ˡ�
��2���ڢߴ����ж��δ�������ԭ���� ��
��3���ݴ�����������Ҫ�ǵ�������������ʱ���徭�����������������Ϊ
��4��20%�ķ������ᣨSO3����������Ϊ20%��1�����ˮ �֣�����2λ��Ч���֣��������ó�98%�ij�Ʒ���ᡣ
��5���ڢڴ�����1500��ġ�����ȫȼ�ա������Ȼ����������������Ȼ���ڢ۴���700�����ټ���ȼ�ա���˵��Ϊ������ȼ�շ�ʽ�Ի��������������ģ� ��
��ѧ��Ӧʽ��ʽδ��ƽ�ľ���1��
��1���Ӵ��� ��2�֣� 2SO2��O2 2SO3��2�֣���V2O5��д�ɡ�������Ҳ�ɣ���������1�֣��ޡ�
2SO3��2�֣���V2O5��д�ɡ�������Ҳ�ɣ���������1�֣��ޡ� �����÷֣� ���¹��̣�2�֣�
�����÷֣� ���¹��̣�2�֣�
��2���÷�ӦΪ���淴Ӧ�����δ�ʹ��δ��Ӧ��SO2������������SO3�����Խ��ͳɱ����ԭ�������ʺͱ���������2�֣������ԭ�������ʡ�1�֣������������� 1�֣�
��3��ͨ�����������������SO3�����϶࣬������SO2�Ĵ�������Ӧ���С���2�֣���SO3�����϶ࡱ1�֣���������SO2�Ĵ�������1�֣�
��4��0.066 ��3�֣����²�0.001���ɣ�
��5���ڸ����£������ĵ�����������Ӧ���ɵ����������Ⱦ�������£��������ﱻ��ԭ������N2���Ի���������������2�֣������ɵ������1�֣����������ﱻ��ԭ��1�֣�
������������������������������һ�㾭���������̣�����¯ȼ�ա��Ӵ������������������գ����ȰѺ���Ŀ�ʯ�ڷ���¯�ڳ��ȼ�գ�Ȼ������SO2ͨ��Ӵ����ڣ��ڸ��¸�ѹ�����������±�����������������Ӧ2SO2��O2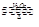 2SO3������Ӧ��Ļ������ͨ����������SO3��ˮ������մӶ��õ��������ᡣ����ͼ�������ʯ�ڷ���¯�Т�ȼ�գ����������ͨ��Ӵ��Ң��У�SO2��O2������SO3�������������ͨ����������SO3��ˮ�����������ᣬ����ͨ��������������գ�Ȼ����ͨ��Ӵ���ʹ�����SO2��һ����������SO3����ͨ����������ˮ���ա�
2SO3������Ӧ��Ļ������ͨ����������SO3��ˮ������մӶ��õ��������ᡣ����ͼ�������ʯ�ڷ���¯�Т�ȼ�գ����������ͨ��Ӵ��Ң��У�SO2��O2������SO3�������������ͨ����������SO3��ˮ�����������ᣬ����ͨ��������������գ�Ȼ����ͨ��Ӵ���ʹ�����SO2��һ����������SO3����ͨ����������ˮ���ա�
��1���ܵ��豸Ϊ�Ӵ��ң���Ӧ�Ļ�ѧ����ʽΪ�� 2SO2��O2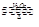 2SO3���÷�Ӧ����ӦΪ���ȷ�Ӧ��Ϊ�����SO3�IJ��ʣ����˲�ȡ������������Ӧ�ò�ȡ���¹��̡�
2SO3���÷�Ӧ����ӦΪ���ȷ�Ӧ��Ϊ�����SO3�IJ��ʣ����˲�ȡ������������Ӧ�ò�ȡ���¹��̡�
��2���÷�ӦΪ���淴Ӧ�����δ�ʹ��δ��Ӧ��ȫ��SO2������������SO3�����Խ��ͳɱ����ԭ�������ʺͱ���������
��3���ݴ�����ΪSO2��O2��SO3�Ļ�����壬���徭����������SO3��ˮ���գ�Ȼ���ٽ�����������ʹδ����ȫ���յ�SO3�ٴα�ˮ������գ�֮���������е�SO3�������٣���ʱ�ٽ���������ٴ�ͨ��Ӵ��Ңߣ���ôSO2��ת���ʻ���SO3�IJ��ʻ�����ߡ�
��4��1�ַ���������SO3����������Ϊ20%������m��SO3��=0.2�֣�m��H2SO4��=0.8�֣���ˮ������Ӧ��SO3 + H2O=H2SO4 ��ʱ��Ҫˮm��H2O��=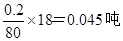 ����������m��H2SO4��=
����������m��H2SO4��=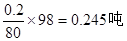 �����ܹ������������Ϊ��m��H2SO4��=0.8+0.245=1.045�֣������ó�98%�ij�Ʒ����ʱ��Ҫˮ������Ϊm��H2O��=
�����ܹ������������Ϊ��m��H2SO4��=0.8+0.245=1.045�֣������ó�98%�ij�Ʒ����ʱ��Ҫˮ������Ϊm��H2O��= ��������Ҫˮ��������m��H2O��=0.045+0.021=0.066�֡�
��������Ҫˮ��������m��H2O��=0.045+0.021=0.066�֡�
��5���ڸ����£������ĵ�����������Ӧ���ɵ����������Ⱦ�������£��������ﱻSO2��ԭ������N2���Ի�������������
���㣺���⿼��������������Ĺ������̡���Ҫ���칤�����̵Ĺ���ԭ��������Ҫ���豸����ҵ�����뻷����������ɫ��ѧ�Ĺ�ϵ����صĻ�ѧ���㡣

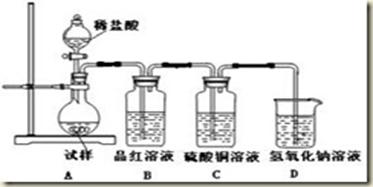
ij��ȤС���������ͼʵ��װ�ý���ʵ�顣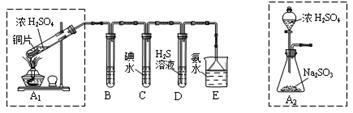
��̽��������Ⱦ��SO2������
��1��Ϊ��ʵ����ɫ������Ŀ�꣬�ܷ�����ͼA2����A1װ�� ����ܡ�����
��2��B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�Ϊ ��C�з�Ӧ�����ӷ���ʽΪ ��D�з�Ӧ�Ļ�ѧ����ʽΪ ��
��̽��ͭƬ��ŨH2SO4��Ӧ�IJ���
ʵ�������������ͭƬ���渽�ź�ɫ���塣�������ϵ�֪���˺�ɫ������ܺ���CuO��CuS��Cu2S��������CuS��Cu2S��������ϡ���ᣬ�ڿ��������ն�ת��ΪCu2O��SO2����С��ͬѧ�ռ�һ������ɫ���壬������ʵ�鷽��̽����ɷ֣�
��3��������м�������ϴ�Ӹɾ���ʵ�鷽����_____________________________��
��4����ɫ����ijɷ���________________��
�������
�ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH-��SO32-��SO42-��HSO3-�������ӡ�
��5����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽΪ ��
��6����֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2 mol/L���ᡢ2 mol/LHNO3��1 mol/LBaCl2��Һ��l mol/LBa(OH)2��Һ��Ʒ����Һ������ˮ�������ʵ��֤��������Һ���д���SO32-��HSO3-������±���ʵ�������Ԥ������ͽ��ۣ�
| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡl mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ� ������Һ���д���SO32-�� SO42-�� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� �� | �� ������Һ���д��� SO32-�� |
| ����3�� �� | �� ������Һ���д��� HSO3-�� |
�ף��������о���ѧϰС��Ϊ�ⶨ�������е�����ԭ�Ӹ����ȣ����������ʵ�����̣�
��ͼA��B��CΪ�ף�����С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��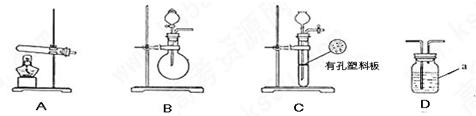
ʵ�鿪ʼǰװ���еĿ������ž�����С���ã���Ӧǰ����ͭ������Ϊ ������ͭ��Ӧ��ʣ����������Ϊ
������ͭ��Ӧ��ʣ����������Ϊ �����ɵ����ڱ�״���µ����
�����ɵ����ڱ�״���µ���� ����С����ϴ��װ��Dǰ������������ɵ����ڱ�״���µ������
����С����ϴ��װ��Dǰ������������ɵ����ڱ�״���µ������
��1��д������a�����ƣ� ��
��2���ף�����С��ѡ���˲�ͬ������ȡ�������뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��ո��С�
| | ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� |
| ��С�� | A | �������ƣ������ | ��Ӧ�Ļ�ѧ����ʽΪ �� |
| ��С�� | �� | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã� �� |
��3����С�����������ݼ�����������е������ԭ�Ӹ���֮��Ϊ ��
��4���ڲ����ͼ�����ȷ������£���С�����������ݼ�����������е������ԭ�Ӹ���������С������ֵ����ԭ������� ��
��5����С����ԭ��ʵ��Ļ�����������һ��װ��ҩƷ��ʵ������������ʵ�飬�ó�������ʵ��������ҩƷ�������� ��
���ü�������������ȡ����Ӧ��ȡ����Ʒ����������ڹ�ҵ���ѳ�Ϊ��ʵ��ij��ѧ��ȤС��ͨ����ʵ������ģ���������̣�����Ƶ�ģ��װ�����£�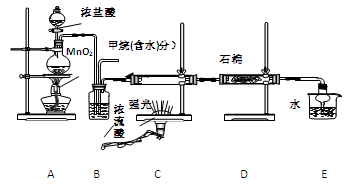
��1�� Bװ�������ֹ��ܣ��ٿ��������ٶȣ��ھ��Ȼ�����壻��
|
��4�� ��Cװ���У�����һ��ʱ���ǿ�����䣬����Ӳ�ʲ������ڱ��к�ɫС����������д���û�����ɫС�����Ļ�ѧ����ʽ ��
��5�� Eװ���г������⣬�������л����E�з�����������ѷ���Ϊ ��
A.��Һ�� B.���� C.��ȡ��Һ�� D.�ᾧ��
��6����װ�û���ȱ�ݣ�ԭ����û�н���β����������β����Ҫ�ɷ�Ϊ �����ţ�
A��CH4 B��CH3Cl C��CH2Cl2 D��CHCl3
���и��������У�����֮��ͨ��һ����Ӧ����ʵ��ͼʾ�仯����
| ���ʱ�� | ����ת����ϵ | a | b | c | d |
| �� |  | FeCl2 | FeC13 | Fe | CuCl2 |
| �� | Mg | MgO | MgCl2 | Mg(OH)2 | |
| �� | NaOH | Na2CO3 | NaHCO3 | NaCl | |
| �� | Al2O3 | NaAlO2 | Al | Al(OH)3 |
A���٢ܡ������� B���ڢܡ������� C���٢ۢܡ������� D���٢ڢۡ�����
��֪X��Y��Z��W��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ��ͼ��ʾ(���ֲ�������ȥ)����W��X��������(����)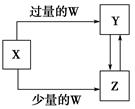
| ѡ�� | W | X |
| A | ���� | Na2CO3��Һ |
| B | ϡHNO3 | Fe |
| C | CO2 | Ca(OH)2��Һ |
| D | O2 | Na |
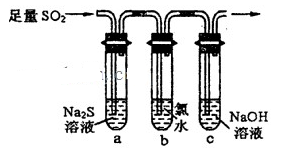
 ���÷�Ӧ�б�������Ԫ����________����Ԫ�ط��ţ���
���÷�Ӧ�б�������Ԫ����________����Ԫ�ط��ţ���