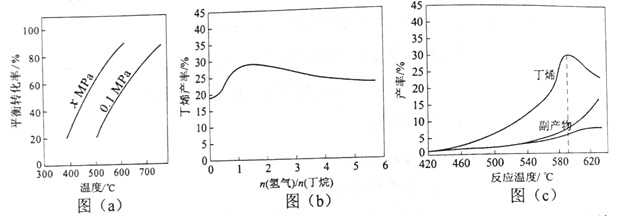
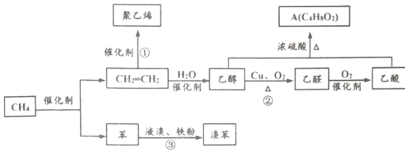
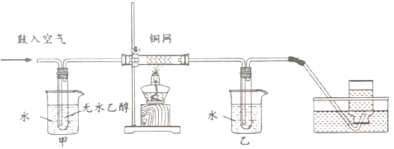
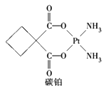
��Ŀ����
����Ŀ��K2Cr2O7��Һ�д���ƽ�⣺Cr2O72-(��ɫ)+H2O2CrO42-(��ɫ)+2H+����K2Cr2O7��Һ��������ʵ�飺���ʵ�飬����˵������ȷ����( )
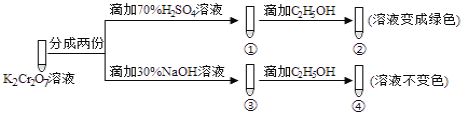
A.������м���70%H2SO4��Һ����������Һ��Ϊ��ɫ
B.����C2H5OH��Cr2O72-������CH3COOH
C.�ԱȢںܿ͢�֪K2Cr2O7������Һ�����Ա�K2CrO4��Һǿ
D.������Һ��ɫ���������Һ���
���𰸡�A
��������
��ͼ�п��Կ���������70% H2SO4��K2Cr2O7��Һ�У�ƽ�������ƶ�����Һ�Գʳ�ɫ������C2H5OH��Һ���ܱ�������CH3COOH��K2Cr2O7����ԭΪCr3+����Һ����ɫ��K2Cr2O7��Һ�м���30%NaOH��Һ��ƽ�������ƶ���K2Cr2O7ת��ΪK2CrO4���ټ���C2H5OH����Һ����ɫ�������K2CrO4���ܽ�C2H5OH������
A��������м���70%H2SO4��Һ��������K2CrO4ת��ΪK2Cr2O7��K2Cr2O7��C2H5OH��ԭ����Һ��Ϊ��ɫ��A����ȷ��
B����ͼ��ʵ���֪������C2H5OH��Cr2O72-����������CH3COOH��B��ȷ��
C���ԱȢںܿ͢�֪��K2Cr2O7������Һ�ܽ�C2H5OH��������K2CrO4��Һ���ܽ�C2H5OH�����������K2Cr2O7�����Ա�K2CrO4ǿ��C��ȷ��
D������ƽ�������ƶ�����Һ��ɫ�������ƽ�������ƶ�����Һ��ƣ�D��ȷ��
��ѡA��

 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�����Ŀ���ƺͼ��Ǽ����õĽ���Ԫ�أ��ƺͼؼ����������������������й㷺��Ӧ�á�
��1��д�����ֿ���ʳ�õĺ��ƻ�����Ļ�ѧʽ��________����0.01 mol��������(��Na2O2����Na2O����Na2CO3����NaCl)�ֱ����100 mL����ˮ�У��ָ������£�������Һ��������Ũ���ɴ�С��˳����(��Һ����仯���Բ���)_______��
��2�����ڼر��Ƹ����ã��Ʊ�K2Oһ�����õ��ʼػ�ԭ��Ӧ�Ĺ�����������λ��������Σ���д���ü�������ط�Ӧ��ȡK2O�Ļ�ѧ����ʽ(����һ�ֵ�������)��______________________��K2O2Ҳ��ǿ�����ԣ���д������SO2������Ӧ�Ļ�ѧ����ʽ��_______________________��
��3��ijѧ����Na2CO3�� KHCO3��ɵ�ij��������ʵ�飬����������(��������ʵ���Ũ������Ҳ�����HCl�Ļӷ�)
ʵ����� | �� | �� | �� | �� |
�������/mL | 50 | 50 | 50 | 50 |
��������/g | 3.06 | 6.12 | 9.18 | 12.24 |
�����������/L(���) | 0.672 | 1.344 | 1.568 | 1.344 |
�������ݼ���������������ʵ���Ũ��Ϊ__________��ԭ�������Ʒ��n(Na2CO3)��n(KHCO3)��_________��