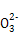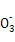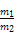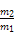ЬтФПФкШн
вбжЊ 0.4 mol вКЬЌыТКЭзуСПH2O2ЗДгІЩњГЩЕЊЦјКЭЫЎеєЦјЪБЗХГі256.64 kJЕФШШСПЁЃ
(1)аДГіыТКЭH2O2ЗДгІЕФШШЛЏбЇЗНГЬЪН: ЁЃ
(2)вбжЊH2O(l)=H2O(g) ІЄH=+44 kJ/mol,дђ16 gвКЬЌыТгызуСПЫЋбѕЫЎЗДгІЩњГЩЕЊЦјКЭвКЬЌЫЎЪБ,ЗХГіЕФШШСПЪЧ ЁЃ
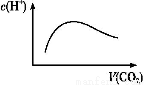
(3)ЩЯЪіЗДгІгІгУгкЛ№М§ЭЦНјЦї,Г§ЪЭЗХГіДѓСПШШСПКЭПьЫйВњЩњДѓСПЦјЬхЭт,ЛЙгавЛИіКмЭЛГіЕФгХЕуЪЧ ЁЃ
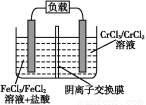
(4)ЯђДЮТШЫсФЦШмвКжаЭЈШывЛЖЈЮяжЪЕФСПЕФАБЦјПЩЩњГЩыТ,аДГіЗДгІЕФРызгЗНГЬЪН: ,ИУЗДгІЕФЛЙдВњЮяЪЧ ЁЃ
(1)N2H4(l)+2H2O2(l) N2(g)+4H2O(g)
N2(g)+4H2O(g)
ІЄH=-641.6 kJ/mol
(2)408.8 kJ
(3)ВњЮяЮЊЕЊЦјКЭЫЎ,ЖдЛЗОГЮоЮлШО
(4)2NH3+ClO- N2H4+Cl-+H2O NaCl
N2H4+Cl-+H2O NaCl
ЁОНтЮіЁП(1)ЪзЯШИљОнЬтвтШЗЖЈЗДгІЮяКЭЩњГЩЮя,ШЛКѓаДГіЛЏбЇЗНГЬЪН:N2H4+2H2O2 N2Ёќ+4H2OЁЃ0.4 molвКЬЌыТКЭзуСПH2O2ЗДгІЩњГЩЕЊЦјКЭЫЎеєЦјЪБЗХГі256.64 kJЕФШШСП,дђ1 molвКЬЌыТКЭзуСПH2O2ЗДгІЪБЗХГіШШСПЮЊ:2.5ЁС256.64 kJ=641.6 kJ,гЩДЫПЩШЗЖЈЩЯЪіЗДгІЕФШШЛЏбЇЗНГЬЪНЕФІЄHЁЃ
N2Ёќ+4H2OЁЃ0.4 molвКЬЌыТКЭзуСПH2O2ЗДгІЩњГЩЕЊЦјКЭЫЎеєЦјЪБЗХГі256.64 kJЕФШШСП,дђ1 molвКЬЌыТКЭзуСПH2O2ЗДгІЪБЗХГіШШСПЮЊ:2.5ЁС256.64 kJ=641.6 kJ,гЩДЫПЩШЗЖЈЩЯЪіЗДгІЕФШШЛЏбЇЗНГЬЪНЕФІЄHЁЃ
(2)16 gвКЬЌыТЕФЮяжЪЕФСПЮЊ0.5 mol,дђгызуСПЫЋбѕЫЎЗДгІЩњГЩ0.5 mol N2КЭ2 mol H2O(l)ЪБЗХГіШШСПЮЊ:0.5 molЁС641.6 kJ/mol+2 molЁС44 kJ/mol=408.8 kJЁЃ
(3)ЗДгІЩњГЩЕФЮяжЪЪЧN2КЭH2O,ЖдЛЗОГЮоЮлШОЁЃ
(4)АБЦјжаЕЊдЊЫиЕФЛЏКЯМлЮЊ-3Мл,ыТЗжзгжаЕЊдЊЫиЕФЛЏКЯМлЮЊ-2Мл,дђАБЦјЪЧЛЙдМС,ДЮТШЫсФЦЪЧбѕЛЏМС,ЛЙдВњЮяЪЧNaCl,ШЛКѓИљОнЕчзгзЊвЦЪиКувдМАЕчКЩЪиКуМДПЩаДГіРызгЗНГЬЪНЁЃ