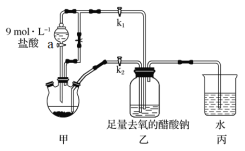
��Ŀ����
����Ŀ��������F��һ��ʳƷ���ʼ����ɰ�����;���ϳɣ�

��֪��RCHO��CH3CHO![]() RCH(OH)CH2CHO��
RCH(OH)CH2CHO��
�Իش�
��1��A�Ļ�ѧ������_________��A��B�ķ�Ӧ������_________��
��2��B��C��Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��3��C��D�����Լ��ͷ�Ӧ�����ֱ���_____________��
��4��E�Ľṹ��ʽ��______________��F�й����ŵ�������________________��
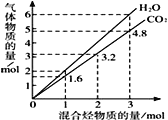
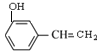
��5������˫��̼�ϵ��ǻ����ȶ�����ת��Ϊ�ʻ�����D��ͬ���칹���У�ֻ��һ�����ķ����廯������______�֡����б�����ֻ��һ��ȡ�������˴Ź���������5���壬�������Ϊ2��1��2��2��1��ͬ���칹��Ľṹ��ʽΪ______________��
��6��д�����Ҵ�Ϊԭ���Ʊ�2-��ϩȩ�ĺϳ�·�ߣ������Լ���ѡ����_____________���ϳ�·������ͼʾ�����£�![]()
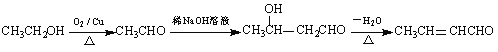
���𰸡��Զ��ױ�����1, 4-���ױ��� ȡ����Ӧ 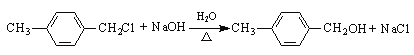 O2 / Cu��Ag�����ȣ�������������������ȣ�
O2 / Cu��Ag�����ȣ�������������������ȣ� 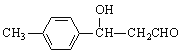 ̼̼˫����ȩ�� 8
̼̼˫����ȩ�� 8 ![]()
![]()
��������
��������ͼ��E��ˮ����![]() ���������Ʒ���E��
���������Ʒ���E��![]() ������RCHO��CH3CHO
������RCHO��CH3CHO![]() RCH(OH)CH2CHO��
RCH(OH)CH2CHO��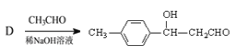 ������D��
������D��![]() ������B�ķ���ʽ
������B�ķ���ʽ![]() �����D�Ľṹ��B��
�����D�Ľṹ��B��![]() ��C��
��C��![]() ����A��
����A��![]() ��
��
�������Ϸ�������1��A��![]() ����ѧ�����ǶԶ��ױ���
����ѧ�����ǶԶ��ױ���![]() ���ϵ���ԭ�ӱ���ԭ�Ӵ�������
���ϵ���ԭ�ӱ���ԭ�Ӵ�������![]() ����Ӧ������ȡ����Ӧ����2��
����Ӧ������ȡ����Ӧ����2��![]() ������������Һ��ˮ��Ϊ
������������Һ��ˮ��Ϊ![]() ����Ӧ�Ļ�ѧ����ʽΪ
����Ӧ�Ļ�ѧ����ʽΪ ����3��
����3��![]() ��ͭ�������������¼������ܱ���������Ϊ
��ͭ�������������¼������ܱ���������Ϊ![]() �������Լ��ͷ�Ӧ�����ֱ���O2 / Cu��Ag�����ȡ���4��E�Ľṹ��ʽ��
�������Լ��ͷ�Ӧ�����ֱ���O2 / Cu��Ag�����ȡ���4��E�Ľṹ��ʽ��![]() ��F��
��F��![]() �����еĹ����ŵ�������̼̼˫����ȩ������5������˫��̼�ϵ��ǻ����ȶ�����ת��Ϊ�ʻ�����D��ͬ���칹���У�ֻ��һ�����ķ����廯������
�����еĹ����ŵ�������̼̼˫����ȩ������5������˫��̼�ϵ��ǻ����ȶ�����ת��Ϊ�ʻ�����D��ͬ���칹���У�ֻ��һ�����ķ����廯������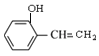 ��
�� ��
��![]() ��
��![]() ��
��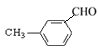 ��
��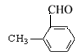 ��
��![]() ��
��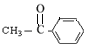 ����8�֡����б�����ֻ��һ��ȡ�������˴Ź���������5���壬�������Ϊ2��1��2��2��1��ͬ���칹��Ľṹ��ʽΪ
����8�֡����б�����ֻ��һ��ȡ�������˴Ź���������5���壬�������Ϊ2��1��2��2��1��ͬ���칹��Ľṹ��ʽΪ![]()
![]() ��
��
��6�����Ҵ�Ϊԭ���Ʊ�2-��ϩȩ���Ȱ��Ҵ�����Ϊ��ȩ��Ȼ��2������ȩ����������ϡ��Һ������![]() �����
�����![]() ��ˮ����2-��ϩȩ���ϳ�·������ͼΪ��
��ˮ����2-��ϩȩ���ϳ�·������ͼΪ�� ��
��

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�