��Ŀ����
����Ŀ�����������ڹ�ҵ�����Ѓӹ㷺��Ӧ�ã�ijͬѧ��ʵ���ж��������ε��Ʊ������ʽ���̽����
(1)Cu2SO3��CuSO32H2O��һ�����ɫ���壬������ˮ���Ҵ���100��ʱ�����ֽ⣬���Ʊ�ʵ��װ����ͼ��ʾ��

������X��������________����������װ��A��ȡSO2ʱ���ý�Ũ�����������ϡ���ᣬ��ԭ����____________________��
��װ��C��������________________________��
��װ��B�з�����Ӧ�����ӷ���ʽΪ_____________________��
����װ��B�л�õĹ�������������ˮ���ϴ�ӣ�����ո������ֱ���ú�ɵķ�ʽ�õ���Ʒ����ԭ����_________________________��
(2)��NaHSO3��Һ�м���NaClO��Һʱ����Ӧ�����ֿ��ܵ������
I.NaHSO3��NaClOǡ�÷�Ӧ��II.NaHSO3������III.NaClO��������ͬѧ��ͨ������ʵ��ȷ���÷�Ӧ������һ�������������±���
ʵ����� | Ԥ�������� |
ȡ������Ӧ��Ļ����Һ���Թ� A�У��μ�ϡ���� | �������ݲ�������_��__(�I����II����III������ͬ)��������û�����ݲ�������_��___���� |
��ȡ������Ӧ��Ļ����Һ���Թ�B�У��μӼ��ε���KI��Һ�� ����� | ��___����III���� |
(3)����Ƽ�ʵ�鷽���Ƚ�������NaHSO3ŨҺ��HSO3-�ĵ���ƽ�ⳣ��Ka��ˮ��ƽ�ⳣ��Kb����Դ�С��________________________��
���𰸡� ��Һ©�� SO2������ˮ���ý�Ũ������������SO2���ݳ� ��ֹ����(������ȫƿ) 3Cu2++3SO2+6H2O==Cu2SO3CuSO32H2O��+8H++SO42- ��ֹCu2SO3CuSO32H2O �����ֽ�ͱ����� II I��III ��Һ��Ϊ��ɫ �����£���pH��ֽ(��pH��)�ⶨNaHSO3��Һ��pH����PH<7����Ka>Kb����pH>7����Ka<Kb
��������(1)�����������Ľṹ��֪����X�������Ƿ�Һ©������������������ˮ����Ũ���Ậˮ���٣�������ˮ�������ڶ�������������
��װ��CΪ��ȫƿ����������
��װ��B������ͭ������������ɲ���Cu2SO3CuSO32H2O�ķ�Ӧ��ͭԪ�ػ��ϼ��н��͵�+1�ۣ��������������Ԫ�����۵�+6�ۣ�������ӷ���ʽΪ��3Cu2++3SO2+6H2O=Cu2SO3CuSO32H2O��+8H++SO42-��
�������֪��л���ն��������ֽ⣬���ܺ����Ϊ�˷�ֹCu2SO3CuSO32H2O �����ֽ�ͱ�������
(2)��NaHSO3��Һ�м���NaClO��Һʱ����Ӧ��I��NaHSO3��NaClOǡ�÷�Ӧ��NaHSO3+NaClO=NaHSO4+NaCl������NaClO���㣺2NaHSO3+NaClO=Na2SO4+SO2��+H2O+NaCl������NaClO������NaHSO3+NaClO=Na2SO4+NaCl+HClO��
��� | ʵ����� | Ԥ�������� |
�� | �������Թ�A�еμ���ˮ������� | ����Һ��ɫ����ϲ���������������������Һ����ɫ����ϲ��������������� |
�� | ��ȡ���������Һ���Թ�B�У��μӼ��ε���KI��Һ������� | ��Һ��Ϊ��ɫ������������ |
(3)���������ԣ�ˮ���Լ��ԣ���ⶨpH���ɣ������ʵ��Ϊ�����£���pH��ֽ����pH�ƣ��ⶨNaHS03��Һ��pH����pH��7����Ka��Kb����pH��7����Ka��Kb��

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�����Ŀ���±���Ԫ�����ڱ���һ����,��Ա��еĢ�����Ԫ�أ���д���пհף�
�� ���� | IA | IIA | ��A | IVA | VA | VIA | ��A |
1 | �� | ||||||
2 | �� | �� | �� | ||||
3 | �� | �� | �� | �� | �� | �� |
(1)����Ԫ���У��γɻ���������������_____________(��Ԫ�ط���)��
(2)Ԫ�آ١��ܺ͢��γɵĻ�����ĵ���ʽ��_______���û������д��ڵĻ�ѧ��������_______��
(3)�ڡ��ۡ�������Ԫ��ԭ�Ӱ뾶�ɴ�С��˳����___________(��Ԫ�ط��ű�ʾ)��
(4)�ݡ��ޡ�������Ԫ�����{���������Ӧˮ����ļ�����ǿ������˳����______________��(�ö�Ӧ���ʵĻ�ѧʽ��ʾ��
(5)Ԫ�آߺ͢�����������Ӧ��ˮ�������Ӧ�����ӷ���ʽΪ___________________��
(6)�ܱȽ�Ԫ�آ�͢�ǽ�����ǿ����ʵ����ʵ��_________(����ĸ���)��
a.����⻯������ԱȢ���⻯���������
b.��ĵ���R2��H2���ϱȢ�ĵ���Q��H2�������ף���HR���ȶ��Ա�H2Qǿ
c.�ڢ���⻯��H2Q��ˮ��Һ��ͨ������ĵ���R2������û�������Q
����Ŀ�����仯�����ڲ������졢�л��ϳɵȷ�����;�dz��㷺���ش��������⣺
(1)VB2��һ�ֵ����մɲ��ϣ���̬��ԭ�ӵļ۵����Ų�ͼΪ_______��
(2)B��C��N����Ԫ�ص�һ��������С�����˳��Ϊ________��
(3)���±�����ڹ�ҵ������Ҫ���ã��������±����ķе����±���ʾ��
BF3 | BCl3 | BBr3 | BI3 | |
�е�/K | 172 | 285 | 364 | 483 |
������±����е��������ߵ�ԭ����__________________��
����BF3���ӽṹ���ͷ�ӦBF3(g)+NH4F(s)==NH4[BF4] (s)�ܹ�������ԭ��____________��
�Ʊ�������ķ������£�
![]()
BCl3��LiBH4����ԭ�ӵ��ӻ������������Ϊ_________����B3N3H6��Ϊ�ȵ�����ķ��ӵĽṹ��ʽΪ________________��
(4)������������۵�Ϊ3000�棬�侧���ṹ��ͼ��ʾ����������a=361.5pm��
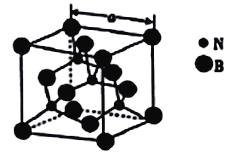
������������ľ�������Ϊ_______________��
�ڽ��ڵ�������ԭ�Ӽ�ľ���Ϊ_______(�г�����ʽ����) pm��
��������������ܶ�Ϊ_____(�г�����ʽ����)g�M-3��




