��Ŀ����
2�� ��50mL 0.50mol/L������50mL 0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL 0.50mol/L������50mL 0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�ǻ��β������������ͼ��֪��װ���в���֮����Ӧ��θ������ڴ�С�ձ�����������ֽ����ʹС�ձ�������ձ�����ƽ��
��2���ձ���������������ĭ�������DZ��¡����ȣ�����ʵ������е�������ʧ��
��3�������60mL 0.50mol/L������50mL 0.55mol/LNaOH��Һ���з�Ӧ������ ��ʵ����ȣ����ų�����������ȣ����ȡ�����ȡ����������к�����ȣ����ȡ�����ȡ��������������к�����ָ�������кͷ�Ӧ����1molˮ���ų�������Ϊ���ģ������ᡢ��������أ�
��4������ͬŨ�Ⱥ�����İ�ˮ��NH3•H2O������NaOH��Һ��������ʵ�飬��õ��к��ȵľ���ֵ��ƫС���ƫ����ƫС��������Ӱ�족����
���� ��1����1���������ȼƵĹ������жϸ�װ�õ�ȱ��������װ�õĴ���
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��4������������ʵ������ȷ�����
��� �⣺��1���������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β����������װ�õĴ����ǣ�С�ձ�������ձ��ڲ���ƽ����δ������ֽ����
�ʴ�Ϊ�����β�����������ڴ�С�ձ�����������ֽ����ʹС�ձ�������ձ�����ƽ��
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹������ձ���������������ĭ�������DZ��¡����ȣ�����ʵ������е�������ʧ��
�ʴ�Ϊ�����¡����ȣ�����ʵ������е�������ʧ��
��3����Ӧ�ų����������������Լ�������Ķ����йأ���60mL 0.50mol/L������50mL 0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ��к�����ֵ��ȣ�
�ʴ�Ϊ������ȣ���ȣ��к�����ָ�������кͷ�Ӧ����1molˮ���ų�������Ϊ���ģ������ᡢ��������أ�
��4��NH3•H2OΪ����������Ϊ���ȹ��̣������ð�ˮ����ϡ����������Һ��Ӧ����Ӧ�ų�������ƫС����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫС��
���� ���⿼��ѧ���й��к��ȵIJⶨ����Ŀ�Ѷ��еȣ�ע�������к��ȵĸ���Ͳⶨԭ���ǽ���Ĺؼ���

| A�� | ����������ʹ��ˮ��ɫ��˵�������������Ư���� | |
| B�� | Ư�ۺ������������ڴ�������ˮ�����ߵ����õ�ԭ����ͬ | |
| C�� | ���������������Ƶõ����������Ƿ��������������������Ը��������Һ | |
| D�� | ��������Na��Al��Fe��һ����������ˮ��Ӧ�����������Ͷ�Ӧ�ļ� |
| A�� | 0��lmo/L��H2S��Һ�У�c��HS-��+c��S2-��=0��lmo/L | |
| B�� | �����£�100 mL pH=2��������10 mL pH=13��NaOH��Һ��ϣ�������ҺpH=7 | |
| C�� | 0��lmo/L CH3COOK��Һ��0��lmo/L KOH��Һ�������ϣ������Һ��Ũ�ȴ�С��ϵ�ǣ�c��K+����c��CH3COO-����c ��OH-����c��CH3COOH�� | |
| D�� | 0��lmo/L��������Һ���٣�NH4��2CO3 �ڣ�NH4��2SO4 �ۣ�NH4��2 Fe��SO4�� 2 ��c��NH4+���Ӵ�С��˳��Ϊ���ۢڢ� |
| A�� | ��NH4��2Fe��SO4��2��Һ�����NaOH��Һ��Ӧ��Fe��OH��2��Fe2++2OH-�TFe��OH��2�� | |
| B�� | ${\;}_{94}^{238}$Pu��${\;}_{92}^{238}$U��Ϊͬλ�� | |
| C�� | H3O+��NH4+���еĵ�������ͬ | |
| D�� | ��BaCl2��Һ����AgNO3��Һ��K2SO4��Һ |
| A�� | �ƹ�ʹ��úҺ���������ɼ��ٶ�����̼������������ŷ� | |
| B�� | ��ǿ��ʯȼ�ϵĿ������ã��ܴӸ����Ͻ����ԴΣ�� | |
| C�� | ���ٻ�����β�����ŷţ����Խ��������ķ��� | |
| D�� | ��ɫʳƷ������ʱ��ʹ�û���ũҩ�������κλ�ѧ���ʵ�ʳƷ |
| A�� | ����ͬϵ������Է������������۵㡢�е������ߣ������µ�״̬����̬�ݱ䵽Һ̬����Է������������Ϊ��̬ | |
| B�� | ����ͬϵ�ﶼ��ʹ��ˮ��KMnO4��Һ��ɫ | |
| C�� | ������±�ص����ڹ����������ܷ���ȡ����Ӧ | |
| D�� | ����ͬϵ����ܶ�����Է����������������� |
| A�� | ����C��ת����Ϊ75% | |
| B�� | ��Ӧ�ﵽƽ��ǰ������ʼ����v������v��������ʼ����v��С��v�� | |
| C�� | �ڸ������£���Ӧ 2C��g��?A��g��+3B��g����ƽ�ⳣ��Ϊ27��1.54��mol/L��2 | |
| D�� | ���е��Ȼ�ѧ����ʽΪ2C��g��?A��g��+3B��g������H=+Q2kJ/mol |
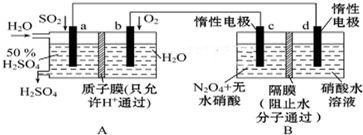
| A�� | �������У�d�缫������Һ��� | |
| B�� | c�缫�ϵĵ缫��ӦʽΪ��2H++2e-=2H2�� | |
| C�� | a������d���� | |
| D�� | �������У�������Ũ�Ȳ��� |