��Ŀ����
14��ij���淴Ӧ��A��g��+3B��g��?2C��g������H��0���м��������ݻ�Ϊ0.5L���ܱ���������������м���1molA�ĺ�3molB��һ�������´ﵽƽ��ʱ�ų�����ΪQ1 kJ������ͬ�����£����������м���2mol C�ﵽƽ��ʱ��������ΪQ2kJ����֪Q2=3Q1��������������ȷ���ǣ�������| A�� | ����C��ת����Ϊ75% | |
| B�� | ��Ӧ�ﵽƽ��ǰ������ʼ����v������v��������ʼ����v��С��v�� | |
| C�� | �ڸ������£���Ӧ 2C��g��?A��g��+3B��g����ƽ�ⳣ��Ϊ27��1.54��mol/L��2 | |
| D�� | ���е��Ȼ�ѧ����ʽΪ2C��g��?A��g��+3B��g������H=+Q2kJ/mol |
���� A���������п�ʼ����1molA�ĺ�3molB���������п�ʼ����2mol CΪ��ȫ��Чƽ�⣬ƽ��ʱ��ͬ��ֵ����ʵ�����ȣ������ƽ��ʱCΪxmol�������з�Ӧ��CΪ��2-x��mol������Q2=3Q1����2-x��mol=3xmol�����x=0.5��������������C��ת���ʣ�
B�����з�Ӧ������н���ƽ�⣬����������н���ƽ�⣻
C����A������֪ƽ��ʱ����CΪ0.5mol���ʣ�
2C��g��?A��g��+3B��g��
��ʼ����mol����2 0 0
�仯����mol����1.5 0.75 2.25
ƽ������mol����0.5 0.75 2.25
�ٸ���K=$\frac{c��A����{c}^{3}��B��}{{c}^{2}��C��}$����ƽ�ⳣ����
D�����淴Ӧ������ȫ��Ӧ��2molC�ֽ����յ���������Q2kJ��
��� �⣺A���������п�ʼ����1molA�ĺ�3molB���������п�ʼ����2mol CΪ��ȫ��Чƽ�⣬ƽ��ʱ��ͬ��ֵ����ʵ�����ȣ������ƽ��ʱCΪxmol�������з�Ӧ��CΪ��2-x��mol������Q2=3Q1����2-x��mol=3xmol�����x=0.5��������C��ת����Ϊ$\frac{��2-0.5��mol}{2mol}$��100%=75%����A��ȷ��
B�����з�Ӧ������н���ƽ�⣬����������н���ƽ�⣬�ʷ�Ӧ�ﵽƽ��ǰ������ʼ����v������v��������ʼ����v��С��v������B��ȷ��
C����A������֪ƽ��ʱ����CΪ0.5mol���ʣ�
2C��g��?A��g��+3B��g��
��ʼ����mol����2 0 0
�仯����mol����1.5 0.75 2.25
ƽ������mol����0.5 0.75 2.25
��ƽ�ⳣ��K=$\frac{c��A����{c}^{3}��B��}{{c}^{2}��C��}$=$\frac{\frac{0.75}{0.5}����\frac{2.25}{0.5}��^{3}}{��\frac{0.5}{0.5}��^{2}}$��mol/L��2=27��1.54��mol/L��2����C��ȷ��
D�����淴Ӧ������ȫ��Ӧ��2molC�ֽ����յ���������Q2kJ����A�з�����֪��2molC��ȫ�ֽ����յ�����ΪQ2kJ��$\frac{2mol}{1.5mol}$=$\frac{4}{3}$Q2kJ����Ӧ�Ȼ�ѧ����ʽΪ��2C��g��?A��g��+3B��g������H=+$\frac{4}{3}$Q2kJ/mol����D����
��ѡ��D��
���� ���⿼���˻�ѧƽ��ļ��㡢��ѧƽ�⽨������ѧƽ�ⳣ���ȣ���Ŀ�Ѷ��еȣ��ؼ��ǵ�Чƽ���Ӧ�ã�ּ������ѧ��������û���֪ʶ���ʵ�������������

| A�� | Na2O��Na2O2���ܺ�ˮ��Ӧ���ɼ���Ƕ��Ǽ��������� | |
| B�� | Na2CO3��Һ��NaHCO3��Һ���ܸ�CaCl2��Һ��Ӧ�õ���ɫ���� | |
| C�� | ���ڳ����²����ױ����� | |
| D�� | ʯ����Һ�м���Na2O2��ĩ���ȱ�������ɫ�������������� |
��1��2P+5CuSO4+8H2O�T5Cu+2H3PO4+5H2SO4
��2��11P+15CuSO4+24H2O�T5Cu3P+6H3PO4+15H2SO4
�����й�˵���д�����ǣ�������
| A�� | ������������Ӧ�У�ˮ�Ȳ���������Ҳ���ǻ�ԭ�� | |
| B�� | ������������Ӧ�У��������ﶼ��H3PO4 | |
| C�� | �ڷ�Ӧ��2���У�����5mol CuSO4������Ӧʱ����ת��10mol���� | |
| D�� | ������������Ӧ�У���������ֻ������ͭ |
 ��50mL 0.50mol/L������50mL 0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL 0.50mol/L������50mL 0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺

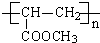 ��
��