��Ŀ����
9����Ҫ��������з���ʽ����1���ö��Ե缫�������ˮ��Һ����д���缫��Ӧʽ���ܷ�Ӧ���ӷ���ʽ��
AgNO3��������4Ag++4e-=4Ag ������4OH--4e-�TO2+2H2O�ܷ�Ӧ���ӷ���ʽ��4Ag++2H2O$\frac{\underline{\;���\;}}{\;}$4Ag+O2+4H+��
��2����Cu�缫�������ˮ��Һ����д���缫��Ӧʽ���ܷ�Ӧ��ѧ����ʽ��
Na2SO4��������2H++2e-�TH2�� ������Cu-2e-=Cu2+�ܷ�Ӧ��ѧ����ʽ��Cu+2H2O$\frac{\underline{\;���\;}}{\;}$Cu��OH��2+H2����
��3����CO2+3H2�TCH3OH+H2O��Ƴ�CO2��H2��H2SO4ԭ��أ���д���缫��Ӧʽ��
������3H2-6e-�T6H+������CO2+6H++6e-=CH3OH+H2O��
���� ��1���ö��Ե缫�����������Һʱ�����������������ӷŵ磬�����������ӷŵ���������
��2����Cu���缫�����������Һʱ�������������ӷŵ�����������������Cuʧ���ӷ���������Ӧ��
��3��CO2��H2��H2SO4ԭ����У�����������ʧ���ӷ���������Ӧ�������϶�����̼�õ������ɼ״���
��� �⣺��1���ö��Ե缫�����������Һʱ�����������������ӷŵ磬�����������ӷŵ���������������������������ط�Ӧʽ�ֱ�Ϊ4Ag++4e-=4Ag��4OH--4e-�TO2+2H2O��4Ag++2H2O$\frac{\underline{\;���\;}}{\;}$4Ag+O2+4H+��
�ʴ�Ϊ��4Ag++4e-=4Ag��4OH--4e-�TO2+2H2O��4Ag++2H2O$\frac{\underline{\;���\;}}{\;}$4Ag+O2+4H+��
��2����Cu���缫�����������Һʱ�������������ӷŵ�����������������Cuʧ���ӷ���������Ӧ��������������������ط�Ӧʽ�ֱ�Ϊ2H++2e-�TH2����Cu-2e-=Cu2+��Cu+2H2O$\frac{\underline{\;���\;}}{\;}$Cu��OH��2+H2�����ʴ�Ϊ��2H++2e-�TH2����Cu-2e-=Cu2+��Cu+2H2O$\frac{\underline{\;���\;}}{\;}$Cu��OH��2+H2����
��3��CO2��H2��H2SO4ԭ����У�����������ʧ���ӷ���������Ӧ�������϶�����̼�õ������ɼ״���������������Ӧʽ�ֱ�Ϊ3H2-6 e-�T6 H+��CO2+6H++6e-=CH3OH+H2O���ʴ�Ϊ��3H2-6 e-�T6 H+��CO2+6H++6e-=CH3OH+H2O��
���� ������ԭ��غ͵���Ϊ���忼��缫��Ӧʽ����д��Ϊ��Ƶ���㣬��ȷ���ӷŵ���д�ǽⱾ��ؼ�����ϵ������Һ�������д�缫��Ӧʽ����Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ֱ������1nm��100nm֮�������Ϊ���� | |
| B�� | ��Ӿ�����֤���������ڵ������Һ | |
| C�� | �������Ӻ�С����������Ĥ | |
| D�� | ���ö����ЧӦ����������Һ�뽺�� |
| A�� | 0.1 �� b-2a �� mol/L | B�� | 10 �� 2a-b �� mol/L | ||
| C�� | 10 �� b-a �� mol/L | D�� | 10 �� b-2a �� mol/L |
| �� | He-268.8 | ��a��-249.5 | Ar-185.8 | Kr-151.7 |
| �� | F2-187.0 | Cl2-33.6 | ��b����58.7 | I2��184.0 |
| �� | ��c����19.4 | HCl-84.0 | HBr-67.0 | HI-35.3 |
| �� | H2O��100.0 | H2S-60.0 | ��d��-42.0 | H2Te-1.8 |
| A�� | ϵ�Тٵģ�a��������û�����Ӽ���Ӧ�й��ۼ��ͷ��Ӽ������� | |
| B�� | ϵ�Т��У�c�����ʵķе��HCl������Ϊ��c���еĹ��ۼ����ι� | |
| C�� | ϵ�Т��У�b�����ʵ�Ԫ�ص�ԭ������ӦΪ35���Ҹ����ʳ�����ΪҺ̬ | |
| D�� | ϵ�Т���H2O�е�仯���ַ���������Ϊ�������������Ӱ�� |
| A�� | 1 mol �� | B�� | 1 mol ���� | C�� | 1 mol ����� | D�� | 1 mol ��ԭ�� |
| A�� | ��ȥ���������е�������������Ҵ���Ũ���ᣬʹ����ȫ��ת��Ϊ�������� | |
| B�� | ��ȥ���е��������ӣ�����NaOH��Һ�������÷ֲ��ȥˮ�� | |
| C�� | ����������Ӧ���Թܣ�����ϡ����ϴ�� | |
| D�� | ��ȡ�ܽ���ˮ�е������⣺����CCl4�������÷ֲ��ȡ���л����ٷ��� |
| A�� | һ���������屩¶�����б�ɺ���ɫ | |
| B�� | ʢ������ɫ�Լ�ƿ�е�Ũ����ʻ�ɫ | |
| C�� | ���μӵ���ˮ�У���ˮ��ӽ���ɫ | |
| D�� | ��������ͨ��Ʒ����Һ����Һ����ɫ |
| A�� | 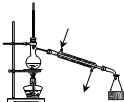 ��������ʯ�� | B�� | 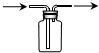 �����ռ�NO | ||
| C�� | 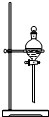 ���ڷ��������������������� | D�� | 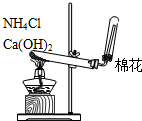 ����ʵ������ȡNH3 |
| A�� | �ʱ䵥λkJ•mol-1����ָ1 mol���ʲμӷ�Ӧʱ�������仯 | |
| B�� | ����Ӧ����ʱ��H��0����Ӧ����ʱ��H��0 | |
| C�� | һ����ѧ��Ӧ�У�����Ӧ��������������������������ʱ����Ӧ���ȣ���HΪ��-�� | |
| D�� | һ����ѧ��Ӧ�У����������ܼ��ܴ��ڷ�Ӧ����ܼ���ʱ����Ӧ���ȣ���HΪ��+�� |