��Ŀ����
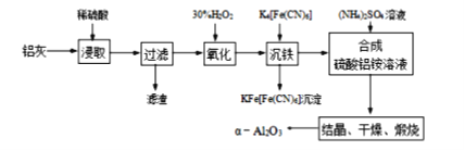
����Ŀ����ұ���ķ���������Ϊԭ����ȡ��ϸa�����������Ƚ��ͻ�����Ⱦ�ֿ��������Դ�������ʡ���֪���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3�������Ʊ�ʵ������ͼ���£�
��1�������������������ᷴӦ�Ļ�ѧ����ʽΪ_______��
��2����30%��H2O2����Ϊ��Fe2+����ΪFe3+,�䷢�������ӷ�Ӧ����ʽΪ__________���÷�Ӧ������¶ȵ���40�棬��Ŀ����_____________��
��3������������茶��壬��������Ҫ��ӦΪ��4[NH4Al(SO4)2��12H2O]��2Al2O3+2NH3��+5SO3��+3SO2��+N2��+53H2O��������������ͨ����ͼ��ʾ��װ�á�
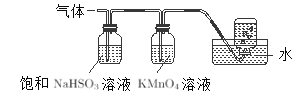
����������NaHSO3��Һ���յ����ʳ���H2O���________���ѧʽ��������ƿ���ռ�����������_______��
��KMnO4��Һ��ɫ��MnO4-��ԭΪMn2+�������������ӷ���ʽΪ_____________��
���𰸡�Al2O3+3H2SO4��Al2(SO4)3+3H2O 2Fe2++H2O2+2H+��2Fe3++2H2O ��ֹH2O2�ֽ� SO3��NH3 N2 2MnO4-+5SO2+2H2O��2Mn2++5SO42-+4H+
��������
�������⣬���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3���������м�ϡ���ᣬAl2O3��FeO��Fe2O3ת��Ϊ���ӣ�SiO2�����������Ϊ���������ˣ���Һ�к���Al3+��Fe2+��Fe3+������˫��ˮ��Fe2+������ΪFe3+������K4[Fe��CN��6]��Fe3+ת��Ϊ���������ˣ�����Һ�м�������泥�����NH4Al��SO4��2���ᾧ��������յõ���-Al2O3���ݴ��жϡ�
��1������������������������������ᷴӦ�Ļ�ѧ����ʽΪAl2O3+3H2SO4��Al2(SO4)3+3H2O��
��2����30%��H2O2����Ϊ��Fe2+����ΪFe3+,˫��ˮ����ԭ����ˮ������ݵ�ʧ�����غ㡢����غ��ԭ���غ���ƽ��������Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+��2Fe3++2H2O������˫��ˮ�����ֽ⣬���Ϊ��ֹH2O2�ֽ⣬�÷�Ӧ������¶ȵ���40�档
��3���ٱ���NaHSO3����SO3��������Ӧ������������NaHSO3��Һ���յ����ʳ���H2O��g���⣬����SO3��NH3��NH4Al��SO4��212H2O�ֽ����ɵ�����NH3��SO3�������������գ������������������,���������ƿ���ռ����������Dz�����ˮ��N2��
�����������£�KMnO4���������Ӧ������������Ӻ������ӣ���Ԫ�ػ��ϼ۴�+7�۽��͵�+2�ۣ��õ�5�����ӣ���Ԫ�ػ��ϼ۴�+4�����ߵ�+6��ʧȥ2�����ӣ�����ݵ�ʧ�����غ㡢����غ㡢ԭ���غ���ƽ���䷴Ӧ�����ӷ���ʽΪ��2MnO4-+5SO2+2H2O��2Mn2++5SO42-+4H+��


