��Ŀ����
������˵������ȷ����____ ��
����ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��
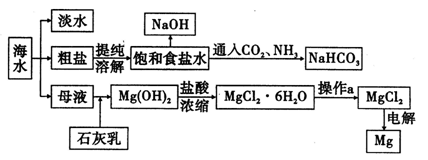
��ش��������⣺
��1�������ӽ���Ĥ��ⱥ��ʳ��ˮʱ�����Ƶı���ʳ��ˮӦ�ü��뵽 ���ҡ�
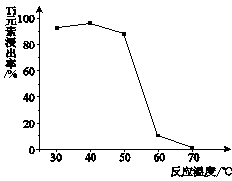
��2����֪�����ӽ���Ĥ�����У�������ÿСʱͨ��1�����ֱ���磬ÿ�ۿ��Բ���1��492 g���ռij������300�����۴�������8Сʱ���Ƶ�32%���ռ���Һ���ܶ�Ϊ1��342��103 kg/m3��113 m3�����۵ĵ���ǿ��1��45��l04 A���õ��۵ĵ��Ч��Ϊ ��
��3��ʾ��ͼ����ȡNaHC03�Ļ�ѧ����ʽΪ ��
��4���������ֱ�Ӽ���Mg��OH��2�õ�Mg0���ٵ������Mg0�ý���Mg�������ɼ����̡����жϸ÷����Ƿ���У���˵������ ��
| A����������ijЩ���ַ����������� |
| B����������Ӳ�ȵ�ˮ��Ҫ�ü��ȵķ������������� |
| C�����Ṥҵ�У��ڽӴ��Ұ�װ�Ƚ�������Ϊ������S03ת��ΪH2S04ʱ�ų������� |
| D���ϳɰ���ҵԭ��������ʱ������̼�����Һ���ճ�ȥ������̼ |

��ش��������⣺
��1�������ӽ���Ĥ��ⱥ��ʳ��ˮʱ�����Ƶı���ʳ��ˮӦ�ü��뵽 ���ҡ�
��2����֪�����ӽ���Ĥ�����У�������ÿСʱͨ��1�����ֱ���磬ÿ�ۿ��Բ���1��492 g���ռij������300�����۴�������8Сʱ���Ƶ�32%���ռ���Һ���ܶ�Ϊ1��342��103 kg/m3��113 m3�����۵ĵ���ǿ��1��45��l04 A���õ��۵ĵ��Ч��Ϊ ��
��3��ʾ��ͼ����ȡNaHC03�Ļ�ѧ����ʽΪ ��
��4���������ֱ�Ӽ���Mg��OH��2�õ�Mg0���ٵ������Mg0�ý���Mg�������ɼ����̡����жϸ÷����Ƿ���У���˵������ ��
��BC��3�֣�
��1������2�֣�
��2��93.46%��3�֣�
��3��NH3+CO2+H2O+NaCl(����)=NaHCO3��+NH4Cl��3�֣�
��4�������У�2�֣���MgO�۵��MgCl2�ߣ�����ʱ���ܸߡ���2�֣�
I��A�����ݸ�����P2O5������ͬ,��������;��ͬ��P2O5�����ϸ�(>10%)�ĸ��������ʣ�P2O5�����ϵ�(4��7%)�ĸ�������������������A��ȷ��B��������ʱӲ�ȵ�ˮ�ſ��ü��ȵķ���������������B����ȷ��C���Ӵ��Ұ�װ�Ƚ�������Ϊ������SO2ת��ΪSO3ʱ�ų�������,C����ȷ��D����ȷ��CO2��H2O��K2CO3=2KHCO3��ѡB C��
��������������ʧ���ӷ���������Ӧ�����Ʊ���ʳ��ˮ���������ң��𰸣�����
�Ʀ�OH�D=��wʵ/w������100%
=��113��32%��1.342��106��/(1.492��1.45��104��300��8)��100%
=48.53/51.92��100%
=93.46%
�𰸣�93.46%
����ͼ�ҳ���Ӧ����������ӦΪ��NH3��H2O��CO2��NaCl�����ͣ�=NaHCO3����NH4Cl���𰸣�NH3��H2O��CO2��NaCl�����ͣ�=NaHCO3����NH4Cl��
��ֱ�ӵ��MgO�������У�MgO�۵�ߣ����ۻ�������ʱ���ܹ��ߡ��𰸣�MgO�۵��MgCl2�ߣ�����ʱ���ܹ��ߡ�
����:��ˮ���ۺ����á���������
��������������ʧ���ӷ���������Ӧ�����Ʊ���ʳ��ˮ���������ң��𰸣�����
�Ʀ�OH�D=��wʵ/w������100%
=��113��32%��1.342��106��/(1.492��1.45��104��300��8)��100%
=48.53/51.92��100%
=93.46%
�𰸣�93.46%
����ͼ�ҳ���Ӧ����������ӦΪ��NH3��H2O��CO2��NaCl�����ͣ�=NaHCO3����NH4Cl���𰸣�NH3��H2O��CO2��NaCl�����ͣ�=NaHCO3����NH4Cl��
��ֱ�ӵ��MgO�������У�MgO�۵�ߣ����ۻ�������ʱ���ܹ��ߡ��𰸣�MgO�۵��MgCl2�ߣ�����ʱ���ܹ��ߡ�
����:��ˮ���ۺ����á���������

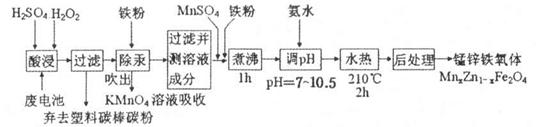
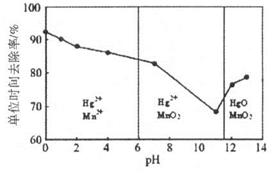
��ϰ��ϵ�д�
�����Ŀ
 2Hg��O2��
2Hg��O2�� 4Al��3O2��
4Al��3O2�� 2Cu��SO2
2Cu��SO2 2Al+3Cl2��
2Al+3Cl2�� Mg+H2O
Mg+H2O Zn+CO2
Zn+CO2 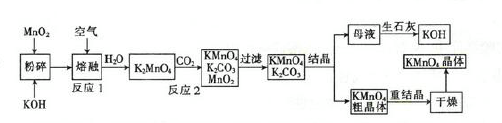

 Li7Ti5O12��3FePO4
Li7Ti5O12��3FePO4 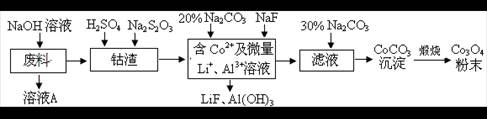
 LiF�����÷�Ӧ��ƽ�ⳣ������ʽΪK= ��
LiF�����÷�Ӧ��ƽ�ⳣ������ʽΪK= �� Mg(OH)2
Mg(OH)2 Mg
Mg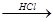 MgCl2��Һ
MgCl2��Һ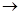 MgCl2����
MgCl2����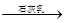 Mg(OH)2
Mg(OH)2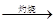 MgO
MgO