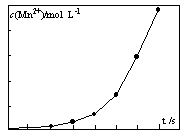
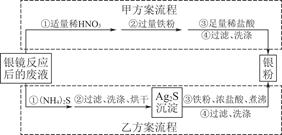
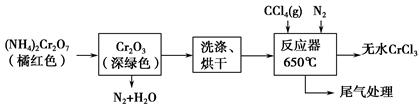
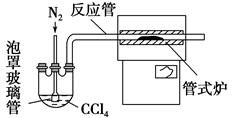
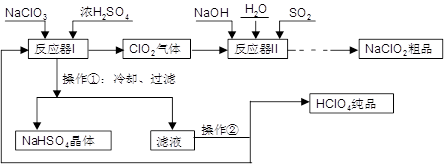
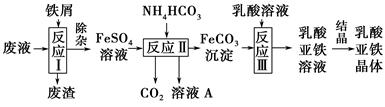
��Ŀ����
�Ȼ�識�ơ���李����ֳ�±ɰ��Ϊ��ɫ������ɫ�ᾧ�Է�ĩ��������ˮ�У��ڹ�ũҵ��������;�㷺�����Ȼ��ƺ������Ϊԭ���Ʊ��Ȼ�識�����Ʒ�����ƣ������������£�

�Ȼ�狀������Ƶ��ܽ�����¶ȱ仯��ͼ��ʾ���ش��������⣺
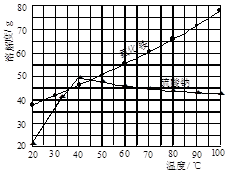
��1��ʵ���ҽ�������Ũ���õ�����Ҫ������ ���ձ������������ƾ��Ƶȡ�
��2��ʵ������г��ȹ��˵�Ŀ���� ��
��3��д��������Ũ����ʱ�����Ļ�ѧ����ʽ�� ��
��4��ij�о���ѧϰС��Ϊ�ⶨ��NH4Cl��Ʒ�е��ĺ������������ͼװ�ã������������ۡ�
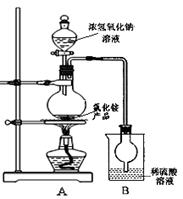
��ͬѧ�����ݴ�ʵ���õ����ݣ������NH4Cl��Ʒ�ĺ���������ƫ�ߣ���Ϊʵ��װ���д���һ������ȱ���ǣ� ____ ��
��ͬѧ��ʵ������У�����ƿ�м����Ũ����������Һ�����ӷ�Ӧ����ʽΪ ����Ӧ������NaOHһ��Ҫ��������ּ��ȣ�ԭ���� ��
�øĽ����ʵ��װ�����½���ʵ�飬��ȡ13.0gNH4Cl��Ʒ�����ʵ���Bװ������3.4g����û��ʺ�����Ϊ ��

�Ȼ�狀������Ƶ��ܽ�����¶ȱ仯��ͼ��ʾ���ش��������⣺
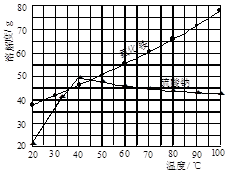
��1��ʵ���ҽ�������Ũ���õ�����Ҫ������ ���ձ������������ƾ��Ƶȡ�
��2��ʵ������г��ȹ��˵�Ŀ���� ��
��3��д��������Ũ����ʱ�����Ļ�ѧ����ʽ�� ��
��4��ij�о���ѧϰС��Ϊ�ⶨ��NH4Cl��Ʒ�е��ĺ������������ͼװ�ã������������ۡ�

��ͬѧ�����ݴ�ʵ���õ����ݣ������NH4Cl��Ʒ�ĺ���������ƫ�ߣ���Ϊʵ��װ���д���һ������ȱ���ǣ� ____ ��
��ͬѧ��ʵ������У�����ƿ�м����Ũ����������Һ�����ӷ�Ӧ����ʽΪ ����Ӧ������NaOHһ��Ҫ��������ּ��ȣ�ԭ���� ��
�øĽ����ʵ��װ�����½���ʵ�飬��ȡ13.0gNH4Cl��Ʒ�����ʵ���Bװ������3.4g����û��ʺ�����Ϊ ��
��1��������
��2����ֹ�Ȼ�茶������������
��3����NH4��2SO4��2NaCl= Na2SO4����2NH4Cl
��4����A��Bװ�ü�ȱһ������װ�� �� NH4++OH- NH3��+H2O
NH3��+H2O
ʹ�Ȼ�麟�ַ�Ӧ��ȫת��ΪNH3 21.5��
��2����ֹ�Ȼ�茶������������
��3����NH4��2SO4��2NaCl= Na2SO4����2NH4Cl
��4����A��Bװ�ü�ȱһ������װ�� �� NH4++OH-
 NH3��+H2O
NH3��+H2O ʹ�Ȼ�麟�ַ�Ӧ��ȫת��ΪNH3 21.5��
�����������1��ʵ���ҽ�������Ũ���õ�����Ҫ�������������ձ������������ƾ��Ƶȣ�
��2����ͼ����Կ�������ijһ�¶ȷ�Χ�ڣ��Ȼ�淋��ܽ�ȵ�������淋��ܽ�ȣ����Գ��ȹ��˷�ֹ�Ȼ�茶�����������ģ�����ʹ������Գ�����ʽ�˳����Ȼ��������Һ�
��3������Ũ��ʱ������Թ�����ʽ���ڣ����Ի�ѧ����ʽΪ��NH4��2SO4��2NaCl= Na2SO4����2NH4Cl
|

��ϰ��ϵ�д�
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ


 3K2MnO4+KCl+3H2O
3K2MnO4+KCl+3H2O 2KMnO4+2KOH+H2������ԭ������ȣ���ⷨ������Ϊ ��
2KMnO4+2KOH+H2������ԭ������ȣ���ⷨ������Ϊ ��