��Ŀ����
| Ԫ�ش��� | �����Ϣ |
| W | W�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������ȣ� |
| X | Xԭ�Ӻ���L�������Ϊ���� |
| Y | Y�ǵؿ��к�������Ԫ�� |
| Z | Z��ͬ����Ԫ�صļ������а뾶��С |
��2��W��X��Y�ĵ�һ�����ܴ�С�����˳����
��3��W��һ���⻯����Է�������Ϊ26��������еĦҼ���м��ļ���֮��Ϊ
��4��X��Z������������Ӧˮ�������Ӧ�����ӷ���ʽΪ
��5����֪�������ݣ�Fe��s��+?O2��g��=FeO��s����H=-272.0KJ?mol-1
4Z��s��+3O2��g��=2Z2O3��s����H=-3351.4KJ?mol-1
Z�ĵ��ʺ�FeO��Ӧ���Ȼ�ѧ����ʽ��
��1��YΪ��Ԫ�أ�λ�����ڱ��ڶ����ڵڢ�A�壬
ZΪΪAlԪ�أ�ԭ�Ӻ��������Ϊ13����̬ԭ�ӵĺ�������Ų�ʽΪ��1s22s22p63s23p1��
�ʴ�Ϊ��������A��1s22s22p63s23p1��
��2��ͬ����������ҵ�һ������������ƣ���NԪ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�������ϵͣ�ʧȥ������Ҫ�������ϴ�һ�����ܸ�������Ԫ�أ��ʵ�һ������C��O��N��
�ʴ�Ϊ��C��O��N��
��3��CԪ�ص�һ���⻯����Է�������Ϊ26��ΪC2H2�������к���1��C��C������2��C-H������������1���Ҽ���2���м����������ǦҼ�����������еĦҼ���м��ļ���֮��Ϊ3��2��
������WY2��CO2��̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ��ʵ���ʽ��
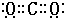 ��
���ʴ�Ϊ��3��2��
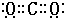 ��
����4��X��Z������������Ӧˮ����ֱ�ΪHNO3��Al��OH��3����Ӧ�����ӷ���ʽΪ��3H++Al��OH��3=Al3++3H2O��
�ʴ�Ϊ��3H++Al��OH��3=Al3++3H2O��
��5����֪���١�Fe��s��+
| 1 |
| 2 |
�ڡ�4Al��s��+3O2��g��=2Al2O3��s����H=-3351.4KJ?mol-1
���ݸ�˹���ɣ��ڡ�
| 1 |
| 2 |
�ʡ�H=
| 1 |
| 2 |
��Al��FeO��Ӧ���Ȼ�ѧ����ʽΪ��2Al��s��+3FeO��s��=Al2O3 ��s��+3Fe��s����H=-859.7KJ/mol��
�ʴ�Ϊ��2Al��s��+3FeO��s��=Al2O3 ��s��+3Fe��s����H=-859.7KJ/mol��

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д�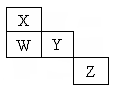 ��ͼΪԪ�����ڱ�ǰ�����ڵ�һ���֣�W��X��Y��Z��Ԫ�����ڱ���ǰ������Ԫ�أ����������ڱ��е����λ���������ʾ�������й���������ȷ���ǣ�������
��ͼΪԪ�����ڱ�ǰ�����ڵ�һ���֣�W��X��Y��Z��Ԫ�����ڱ���ǰ������Ԫ�أ����������ڱ��е����λ���������ʾ�������й���������ȷ���ǣ�������| A��ZԪ��һ���ǽ���Ԫ�� | B��X��Y��Wһ��������Ԫ�� | C��W���⻯��ķе�һ����X���⻯��ķе�� | D��Z�ȿ����������ڣ�Ҳ������������ |
W��X��Y��Z��Ԫ�����ڱ���ԭ��ǰ�����ڵ�����Ԫ�أ��й����ǵ���Ϣ���±���ʾ��
Ԫ�� | �����Ϣ |
�� | W�Ļ�̬ԭ��L���������K���������3�� |
�� | X����Ԫ���γɵ�һ�ֻ��������ʹʪ��ĺ�ɫʯ����ֽ���� |
�� | ����Ϊ����ɫ���壬����������Ӧ��ˮ���ﻯѧʽΪH��O4 |
�� | Z�Ļ�̬ԭ����Χ�����Ų�ʽΪ(n��1)d10ns1 |
��1��Yλ��Ԫ�����ڱ���________���ڵ�________�壻X�Ļ�̬ԭ�Ӻ�������Ų�ʽ��??????????? ��
��2��W�ļ����Ӱ뾶???? X�ļ����Ӱ뾶������>������<������=������Y�ĵ�һ�����ܱ�Z��???? ��������������С������W��X�������̬�⻯���У��е�ϸߵ���??????? ���ѧʽ����
��3����150������ʱ��������ZW��������Ӧ���ɺ�ɫ��Z2W��ĩ���÷�Ӧ�Ļ�ѧ����ʽΪ??????? ��
��4��WԪ���γɵĶ��ֻ���������У����м��Թ��ۼ��ͷǼ��Թ��ۼ��ķ�������Ϊ
???????????????????????? ����дһ�֣���
��5����25����101 kPa�£���֪Z���嵥����Y2��������ȫȼ�պ�ָ���ԭ״̬��ƽ��ÿת��1mol ���ӷ���110.05kJ���÷�Ӧ���Ȼ�ѧ����ʽ��?????? ? ???? ???????????????????????????????? ��
