��Ŀ����
����Ŀ����Fe3�����������ԣ�ʵ���Ҳⶨ�����Һ��I�����������������£���ȡ25.00 mL��Һ��250 mL��ƿ�У��ֱ����5 mL 2 mol��L��1 H2SO4��10 mL 20% Fe2(SO4)3��Һ��ҡ����С���������������ȫ������ȡ����ƿ��ȴ���뼸�ζ����������ƣ�����ָʾ��������0.02500 mol��L��1��K2Cr2O7��Һ���еζ����յ㡣�ظ�3�Σ����ݼ�¼���±���
���� | 1 | 2 | 3 |
����/mL | 19.98 | 20.02 | 19.00 |
��1����ʢ�з�Һ����ƿ���ȼ���5 mL 2 mol��L��1 H2SO4��Ŀ����
______________________________���������ӷ���ʽ�������������ͣ�
��2�������������漰�ķ�Ӧ����2Fe3����2I��===2Fe2����I2 ��______________________________��
��3�����ݵζ��й����ݣ��÷�Һ��I��������_____g��L��1��
��4���ڵζ������У����в���������������ȷ������ɲⶨ���ƫ�͵���___________��
A���յ����ʱ���Ӷ������ζ�ǰƽ�Ӷ���
B����ƿˮϴ�º�δ����
C���ζ���δ�ñ�K2Cr2O7��Һ��ϴ
D��ʢ��K2Cr2O7��Һ�ĵζ��ܣ��ζ�ǰ�����ݣ��ζ���������
��Fe3����Ag�������������ǿ��һֱ��ʵ��̽�����ȵ㡣ijѧϰС��ͬѧ�������ʵ�飺
ʵ���� | ʵ����� | ���� |
1 | ��10mL 3mol/L KNO3������Һ��pH=1���в���һ���ྻ��Ag˿�����μ�NaCl��Һ | �ް�ɫ�������� |
2 | ��10mL 1mol/L AgNO3��Һ�еμ�2mL 0.1mol/L FeSO4��Һ�����ٵμ�����KMnO4��Һ | �Ϻ�ɫ����ȥ |
3 | ��10mL 1mol/L Fe(NO3)3������Һ��pH=1���в���һ���ྻ��Ag˿�����μ�NaCl��Һ | �а�ɫ�������� |
��ش�
��5�����ʵ��ٵ�Ŀ����______________________________��
��6��ʵ��ۿɵó�������______________________________��
��7��д��ʵ����з�Ӧ�����ӷ���ʽ______________________________��
��8����������ʵ�飬Fe3����Ag�������������ǿ��������____________________�йء�
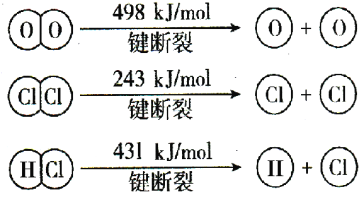
���𰸡���1��Fe3����3H2O![]() Fe(OH)3��3H��������H2SO4ƽ�������ƶ�������Fe3��ˮ��
Fe(OH)3��3H��������H2SO4ƽ�������ƶ�������Fe3��ˮ��
��2��6Fe2����Cr2O72����14H��===6Fe3����2Cr3����7H2O
��3��15.24
��4��A
��5���ų�NO3���ĸ���
��6��Fe3��������Ag
��7��Fe2����Ag��![]() Fe3����Ag��д��=��Ҳ���֣�
Fe3����Ag��д��=��Ҳ���֣�
��8��Ũ��
��������
���������
��1�����Ȼ�ٽ������ӵ�ˮ�⣬����ʢ�з�Һ����ƿ���ȼ���H2SO4��Ŀ�������������ӵ�ˮ�⣻
��2���������֪������K2Cr2O7��Һ�ζ��DzⶨFe2���ĺ������ʷ����ķ�ӦΪ��6Fe2����Cr2O72����14H��===6Fe3����2Cr3����7H2O��
��3���ɷ�Ӧ��2Fe3����2I��=2Fe2����I2 ��6Fe2����Cr2O72����14H��=6Fe3����2Cr3����7H2O��֪��
6I����Cr2O72�������е�3���������ϴ���ȥ����V(K2Cr2O7)=(19.98+20.02)��2=20.00mL��
n(K2Cr2O7)=20.00mL��10��3��0.02500 mol��L��1=0.5��10��3mol��n(I��)=6��0.5��10��3mol=3��10��3mol���ʷ�Һ��I���ĺ���Ϊ127��3��10��3��0.025��10��3=15.24 g��L��1��
��4��A��ζ��յ�ʱ���Ӷ������ζ�ǰƽ�Ӷ���������ɱ�Һ���ƫС�����²ⶨ���ƫ�ͣ�B��Բⶨ�����Ӱ�죻C���Һ��ϡ�ͣ���ɵζ�ʱ�������ƫ���ʹ�ⶨ���ƫ�ߣ�D���Һ�ζ�ǰ�����ݣ��ζ��������ݣ�ʹ��Һ��ȡ���ƫ��ʹ�ⶨ���ƫ�ߡ���ѡA��
��5�����ʵ��ٵ�Ŀ�����ų�NO3���ĸ�����
��6��ʵ��ۿɵó�������Fe3��������Ag��
��7��ʵ����з�Ӧ�����ӷ���ʽΪFe2����Ag��![]() Fe3����Ag��
Fe3����Ag��
��8��Fe3����Ag�������������ǿ��������Ũ���й���

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�