��Ŀ����
����Ŀ��������Ȼ���й㷺�ֲ���Ԫ��֮һ�����ڷ������⻯ѧ���ʣ���������±���ڵ��ʼ���������Ʊ��������ϴ��ڽ����ԵIJ��졣
��.��ѧ���о����֣�SbF5�ܽ�MnF4������[MnF6]2�������з�Ӧ�õ���SbF5ת�����ȶ�����[SbF6]�����Ρ���MnF4�ܲ��ȶ��������ֽ�ΪMnF3��F2�����������о�д����K2MnF6��SbF5Ϊԭ�ϣ��� 423 K ���¶����Ʊ�F2�Ļ�ѧ����ʽ��_________________________��

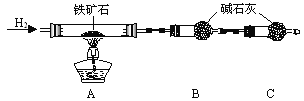
�ִ���ҵ�Ե�����ڵķ��⻯��(KHF2)�ͷ�����(HF)������Ʊ������ʣ�����Ʒ�װ����ͼ��ʾ��
��֪KHF2��һ����ʽ�Σ�д�������Ϸ����ĵ缫��Ӧʽ________________________������Ʒ�ʱ��Ҫ����ͭ�Ͻ���彫������������ϸ�ֿ���ԭ����___________��
��.��±������۷е�����Է����������Ӷ����ߣ���HF�۷е����HCl��ԭ����________________________��HF��ˮ��Һ������ᣬ������ʴ�̲������仯ѧ��Ӧ����ʽΪ��________________________
����֪25 ��ʱ�������(HF)�ĵ���ƽ�ⳣ��Ka��3.6��10��4��
ijpH��2���������Һ����ˮ�������c(H��)��___________mol/L������0.01 mol/L HF��Һ��pH��12��NaOH��Һ�������ϣ�����Һ������Ũ�ȴ�С��ϵΪ��________________________��
������֪25 ��ʱ���ܶȻ�����Ksp(CaF2)��1.46��10��10������1 L 0.2 mol/L HF��Һ�м��� 1 L 0.2 mol/L CaCl2 ��Һ��ͨ����ʽ����˵���Ƿ��г���������______________________
���𰸡� 2K2MnF6��4SbF5![]() 4KSbF6��2MnF3��F2�� _2HF2-��2e��===H2����4F�� �����������ܷ������ҷ�Ӧ��������ը HF���Ӵ��ڷ��Ӽ���� SiO2��4HF===SiF4����2H2O 10��12 c(Na��)>c(F��)>c(OH��)>c(H��) c(H��)��c(F��)��
4KSbF6��2MnF3��F2�� _2HF2-��2e��===H2����4F�� �����������ܷ������ҷ�Ӧ��������ը HF���Ӵ��ڷ��Ӽ���� SiO2��4HF===SiF4����2H2O 10��12 c(Na��)>c(F��)>c(OH��)>c(H��) c(H��)��c(F��)��![]() _mol��L��1��6��10��3_mol��L��1��c(Ca2��)��0.1_mol��L��1��c2(F��)��c(Ca2��)��3.6��10��5��0.1��3.6��10��6>1.46��10��10������ϵ��CaF2����
_mol��L��1��6��10��3_mol��L��1��c(Ca2��)��0.1_mol��L��1��c2(F��)��c(Ca2��)��3.6��10��5��0.1��3.6��10��6>1.46��10��10������ϵ��CaF2����
����������.��K2MnF6��SbF5Ϊԭ�ϣ��� 423 K���¶��£�K2MnF6��SbF5������KSbF6��MnF4��MnF4�����ֽ�ΪMnF3��F2��������Ӧ���ܻ�ѧ����ʽ2K2MnF6��4SbF5![]() 4KSbF6��2MnF3��F2����
4KSbF6��2MnF3��F2����
������H+(��Դ��HF2-)������ԭ��Ӧ������������缫��ӦʽΪ2HF2-��2e��===H2����4F���������������ܷ������ҷ�Ӧ��������ը��Ϊ��ֹ�����������ķ�Ӧ������Ʒ�ʱ��Ҫ����ͭ�Ͻ���彫������������ϸ�ֿ���
��.��HF���Ӽ������������۷е����Ը���HCl��HF��ˮ��Һ������ᣬ���ܽ�SiO2��������ʴ�̲�����������Ӧ�Ļ�ѧ��Ӧ����ʽΪSiO2��4HF===SiF4����2H2O��
��ijpH��2���������Һ��ˮ�ĵ����ܵ����ƣ���ˮ�������c(H��)ˮ��c(OH-)��Һ=![]() =
=![]() mol/L=10��12 mol/L����0.01 mol/L HF��Һ��pH��12��NaOH��Һ�������ϣ�ǡ����ȫ�кͣ�������ҺΪNaF��Һ����F-��ˮ�⣬��Һ�Լ��ԣ���ϵ���غ�ʽ��֪��Һ������Ũ�ȴ�С��ϵΪc(Na��)>c(F��)>c(OH��)>c(H��) ��
mol/L=10��12 mol/L����0.01 mol/L HF��Һ��pH��12��NaOH��Һ�������ϣ�ǡ����ȫ�кͣ�������ҺΪNaF��Һ����F-��ˮ�⣬��Һ�Լ��ԣ���ϵ���غ�ʽ��֪��Һ������Ũ�ȴ�С��ϵΪc(Na��)>c(F��)>c(OH��)>c(H��) ��
������Һ��Ϻ�c(Ca2+)=0.1 molL-1��c(F-)=![]() =
=![]() mol��L��1��6��10��3mol��L��1��c(Ca2��)��0.1mol��L��1����Qc=c2(F-)c(Ca2+)=(3.6��10-5mol/L)0.1 molL-1=3.6��10-6��Ksp��˵���г���������
mol��L��1��6��10��3mol��L��1��c(Ca2��)��0.1mol��L��1����Qc=c2(F-)c(Ca2+)=(3.6��10-5mol/L)0.1 molL-1=3.6��10-6��Ksp��˵���г���������