��Ŀ����
2013�����ȫ�����ض�����ж�������ʮ������������ɡ������족����Ҫ��Դ֮һ������β����ȼúβ���ŷų����Ĺ���С������
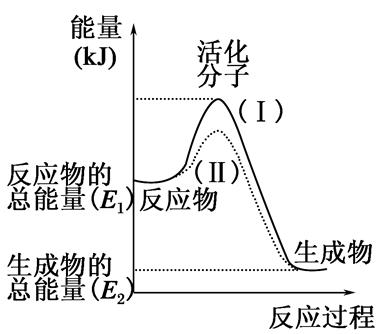
����β����������Ҫԭ��Ϊ��2NO(g)+2CO(g)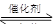 2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ�
2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ� 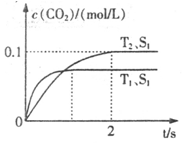
��1���÷�ӦΪ ��Ӧ(����ȡ������ȡ�������T2�¶��£�0~2s�ڵ�ƽ����Ӧ���ʣ�v(N2)= ����2�����������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2���ڴ���ϻ��� c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
��3��ij���л�������t1���£�����㶨���ܱ������У������崫��������˲�ͬʱ���NO��CO��Ũ�ȣ��������ݼ��±���CO2��N2����ʼŨ��Ϊ0����
| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c(NO)/xl0-4mol L-1 | 10��0 | 4��50 | 2��50 | 1��50 | 1��00 | 1��00 |
| c(CO)/xl0-3mol L-1 | 3��60 | 3��05 | 2��85 | 2��75 | 2��70 | 2��70 |
t1��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��ƽ��ʱNO���������Ϊ ��
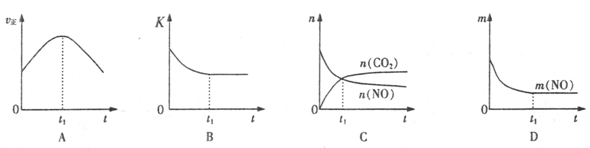
��4�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬���� (����ţ�������ͼ��v����K��n��m�ֱ��ʾ����Ӧ���ʡ�ƽ�ⳣ�������ʵ�����������
��5��úȼ�ղ���������Ҳ�������������CH4����ԭNOX�������������������Ⱦ��
��֪��CH4(g)+2NO2(g) = N2 (g)+CO2 (g)+2H2O(g) ��H=-867��0kJ ? mol-1
2NO2 (g)
 N2O4 (g) ��H=-56��9kJ ? mol-1
N2O4 (g) ��H=-56��9kJ ? mol-1H2O(g) = H2O(l) ��H=-44��0kJ ? mol-1
д��CH4����ԭN2O4 (g)����N2 (g)��CO2 (g)��H2O(l)���Ȼ�ѧ����ʽ ��
��1������ 0��025 mol/(L��s) ����2�֣���4�֣�
��2����ͼ����T1S1�·�����㲻�䡢�յ������ߺɣ��������ɣ���2�֣�

��3��5000 L/mol ��2�֣� 2��41% (2��)
��4��B D ����2�֣���ѡ��1�֣���ѡ���÷֣�
��5��CH4(g)+N2O4(g)�TN2(g)+CO2(g)+2H2O(g) D H�T ��898��1kJ/mol ��2�֣�
������������������ݡ��ȹ���ƽ����ֵ��ԭ��֪��T1��T2������T1�����¿�֪��������̼�ĺ��������ͣ���֪�������¶ȷ�Ӧ�����ƶ�����������Ƿ��ȵġ���ѧ��Ӧ���ʵ��ڵ�λʱ���ڷ�Ӧ�����������Ũ�ȵı仯������˿���ȷ���ö�����̼��ʾ�Ļ�ѧ��Ӧ���ʾ�Ϊ��0.050 mol/(L��s)�������ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ���֪���õ�����ʾ�Ļ�ѧ��Ӧ���ʾ�Ϊ��0.025 mol/(L��s)��������������ֻ������Ӧ���ʣ�����Ӱ�������̼�ĺ�������ˣ�ֻ�Ƿ�Ӧ�ﵽƽ���ʱ���ӳ�������Ӱ�������̼������
�� 2NO(g) + 2CO(g) 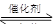 2CO2 + N2
2CO2 + N2
��ʼŨ�ȣ�10��3mol/L 3.6��10��3mol/L 0 0
ת��Ũ�ȣ�9.0��10��4mol/L 9.0��10��4mol/L 9.0��10��4mol/L 4.5��10��4mol/L
ƽ��Ũ�ȣ�10��4mol/L 2.7��10��3mol/L 9.0��10��4mol/L 4.5��10��4mol/L
���ƽ�ⳣ����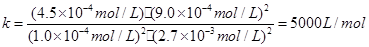
��駣�ƽ��ʱ����ȵ������ʵ���֮�ȣ�����Ũ��֮�ȣ�����ƽ��ʱNO���������Ϊ��

��Aͼ������Ӧ���ʷ�Ӧ��ʼ���Ǽ�С�ģ�����Bͼ�б�ʾ���ǻ�ѧƽ�ⳣ����ʱ��ı仯����Ϊ�Ǿ��ȵģ�����Ӧ���Ƿ��ȵģ��������ŷ�Ӧ�Ľ��У��ų�������ɢ����ȥ��ʹ��Ӧ�����ƶ���ʹ��ƽ�ⳣ����С������ijһ��ʱ�̴ﵽƽ��״̬����ȷ��Cͼ����t1����Ե����ʵ������ڱ仯������ƽ��״̬������Dͼ��һ��������������t1ʱ���������䣬һ����һ��ƽ��״̬����ȷ��
�� ��CH4(g)+2NO2(g) = N2 (g)+CO2 (g)+2H2O(g) ��H=-867��0kJ ? mol-1
��2NO2 (g)  N2O4 (g) ��H=-56��9kJ ? mol-1
N2O4 (g) ��H=-56��9kJ ? mol-1
��H2O(g) = H2O(l) ��H=-44��0kJ ? mol-1
�٣��ڣ��ۡ�2��CH4(g)+N2O4(g)�TN2(g)+CO2(g)+2H2O(g) D H�T ��898��1kJ/mol
���㣺���黯ѧƽ�⣬�Ȼ�ѧ���й�֪ʶ��

�״���һ�ֿ�����������ȼ�ϣ���;�㷺���о������þ��й���ǰ����
��1����֪�ڳ��³�ѹ�£���÷�Ӧ�ķ�Ӧ�����£�
�� 2CH3OH(l)+ 3O2(g)  2CO2(g) +4H2O(g) ?H1= ��1275��6 kJ/mol
2CO2(g) +4H2O(g) ?H1= ��1275��6 kJ/mol
�� 2CO(g) +O2(g)  2CO2(g) ?H2=��566��0 kJ/mol
2CO2(g) ?H2=��566��0 kJ/mol
CH3OH����ȫȼ������CO����̬ˮ���Ȼ�ѧ����ʽ�� ��
��2����ҵ�������״��ķ�Ӧ���£�CO2(g) + 3H2(g)  CH3OH(g)+ H2O(g) ?H = ��49 kJ/mol
CH3OH(g)+ H2O(g) ?H = ��49 kJ/mol
��ij�¶��£��ݻ���Ϊ1 L��A��B���������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��º��ݡ�����B�о�10 s��ﵽƽ�⡣�ﵽƽ��ʱ���й��������±���
| ���� | A | B |
| ��Ӧ��Ͷ���� | 1 mol CO2(g)��3 mol H2(g) | 1 mol CH3OH(g)��1 mol H2O(g) |
| ��Ӧ�����仯 | �ų���kJ���� | ����19��6 kJ���� |
�ٴӷ�Ӧ��ʼ���ﵽƽ��ʱ������B��CH3OH��ƽ����Ӧ����Ϊ ��
�ڸ��¶��£�B�����з�Ӧ�Ļ�ѧƽ�ⳣ������ֵΪ ��
�ۦ��� ��
�����д�ʩ��ʹ����A�м״��IJ���������� ��
a�������¶� b����ˮ��������ϵ����
c���ø���Ч�Ĵ��� d�����������ݻ���Сһ��
��3���ҹ���ѧԺ��ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ء��״�ȼ�ϵ�صĹ���ԭ������ͼ��ʾ��
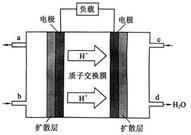
�� �õ�ع���ʱ��b��ͨ�������Ϊ ��
�� �õ�������ĵ缫��ӦʽΪ ��
��ú��Ϊȼ�Ͽ�ͨ����������;����
;��I�� C(s)��O2(g) = CO2(g)
;��II������ˮú���� C(s) + H2O(g) =" CO(g)" + H2(g)
ȼ��ˮú����2 CO(g) + O2(g) = 2CO2(g)��
2H2(g)+O2(g) =2H2O(g)
��֪����C(s)��O2(g)=CO2(g)����H1=��393.5 kJ��mol-1
��H2(g)+1/2O2(g)=H2O(g)����H2=��241.8kJ��mol-1
��CO(g)+ 1/2O2 (g) =CO2(g)����H3=��283.0kJ��mol-1
��ش��������⣺
��1��CO(g) + H2O(g) = H2(g) + CO2(g) �� ������ȷ�Ӧ�������ȷ�Ӧ����
��2�����ݸ�˹���ɣ�ú����̬ˮ����ˮú���ķ�Ӧ�ȡ�H= ��
��3����������;��������˵��������ǣ� ��
| A��;��II��ˮú��ʱ�����ܺģ���;��II����������ȡ |
| B����;��I��ȣ�;��II���Լ��ٶԻ�������Ⱦ |
| C����;��I��ȣ�;��II�������ú��ȼ��Ч�� |
| D����úת��Ϊˮú������ͨ���ܵ��������� |
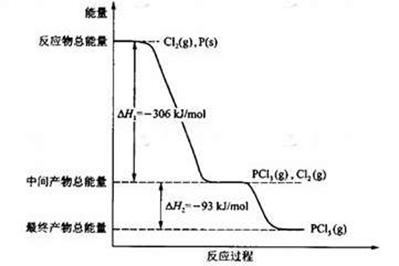
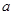 1���� ������Ӧ�¶���T1���ߵ�T2��ƽ��ʱPCl5�ķֽ���Ϊ
1���� ������Ӧ�¶���T1���ߵ�T2��ƽ��ʱPCl5�ķֽ���Ϊ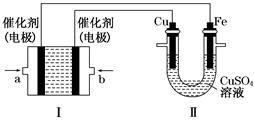
 CO2(g)����H1<0��
CO2(g)����H1<0�� CO(g)��H2(g)��
CO(g)��H2(g)�� O2(g)=H2O(g)����H2����242.0 kJ��mol��1
O2(g)=H2O(g)����H2����242.0 kJ��mol��1 CO2(g)��H2O(g)
CO2(g)��H2O(g)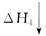

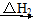 CO(g)��H2O(g)��
CO(g)��H2O(g)��