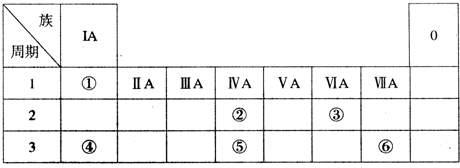
��Ŀ����
���ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺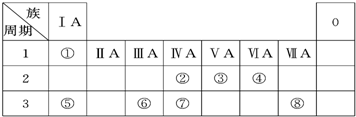
��1��д���ɢܡ��ݡ���Ԫ�����γɵļȺ����Ӽ��ֺ����ۼ���һ�����ӻ�����ĵ���ʽ ��
��2����Ԫ�آ�ĵ�����������ˮ���л��ﷴӦ���ɶ�������Σ�������ʣ���������̭�����пɴ�����������ˮ����������
A��NH2Cl B��AlCl3 C��K2FeO4 D��ClO2
��3��W��������ڵ�ͬ����Ԫ�أ����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
��4���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��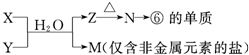
д��X��Һ��Y��Һ��Ӧ�����ӷ���ʽ ��
M�������ӵļ������� ��
���M�к���Ԫ�آ࣬M��Һ������Ũ���ɴ�С������˳���� ��

��1��д���ɢܡ��ݡ���Ԫ�����γɵļȺ����Ӽ��ֺ����ۼ���һ�����ӻ�����ĵ���ʽ
��2����Ԫ�آ�ĵ�����������ˮ���л��ﷴӦ���ɶ�������Σ�������ʣ���������̭�����пɴ�����������ˮ����������
A��NH2Cl B��AlCl3 C��K2FeO4 D��ClO2
��3��W��������ڵ�ͬ����Ԫ�أ����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | ������ | H2WO3+3H3PO3�T3H3PO4+H2W�� |
| 1 | ||
| 2 |

д��X��Һ��Y��Һ��Ӧ�����ӷ���ʽ
M�������ӵļ�������
���M�к���Ԫ�آ࣬M��Һ������Ũ���ɴ�С������˳����
��������Ԫ�������ڱ��е�λ�ÿ�֪��ΪHԪ�أ���ΪCԪ�أ���ΪNԪ�أ���ΪSԪ�أ���ΪNaԪ�أ���ΪAlԪ�أ���ΪSiԪ�أ���ΪClԪ�أ�
��1���ɢܡ��ݡ���Ԫ�����γɵļȺ����Ӽ��ֺ����ۼ���һ�����ӻ�����ΪNaClO��
��2���ɴ�������������ˮ������������Ӧ���������ԣ�
��3��W��������ڵ�ͬ����Ԫ���к����ᣬ��ΪS����Ӧ��H2SO3���������ԡ���ԭ�Ժ����ԣ�
��4����ΪAl����ת����ϵ��֪NΪAl2O3��ZΪAl��OH��3��XΪAlCl3��YΪNH3��MΪNH4Cl��
��1���ɢܡ��ݡ���Ԫ�����γɵļȺ����Ӽ��ֺ����ۼ���һ�����ӻ�����ΪNaClO��
��2���ɴ�������������ˮ������������Ӧ���������ԣ�
��3��W��������ڵ�ͬ����Ԫ���к����ᣬ��ΪS����Ӧ��H2SO3���������ԡ���ԭ�Ժ����ԣ�
��4����ΪAl����ת����ϵ��֪NΪAl2O3��ZΪAl��OH��3��XΪAlCl3��YΪNH3��MΪNH4Cl��
����⣺��Ԫ�������ڱ��е�λ�ÿ�֪��ΪHԪ�أ���ΪCԪ�أ���ΪNԪ�أ���ΪSԪ�أ���ΪNaԪ�أ���ΪAlԪ�أ���ΪSiԪ�أ���ΪClԪ�أ�
��1���ɢܡ��ݡ���Ԫ�����γɵļȺ����Ӽ��ֺ����ۼ���һ�����ӻ�����ΪNaClO������ʽΪ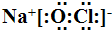 ���ʴ�Ϊ��
���ʴ�Ϊ��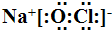 ��
��
��2���ɴ�������������ˮ������������Ӧ���������ԣ�A��ClԪ��Ϊ+1�ۣ���ˮ������HClO��C��D������ǿ�����ԣ�������ɱ���������ʴ�Ϊ��ACD��
��3��W��������ڵ�ͬ����Ԫ���к����ᣬ��ΪS����Ӧ��H2SO3���������ԡ���ԭ�Ժ����ԣ��ɱ���������H2SO4������Ϊ��ԭ�ԣ�����ʽΪ2H2SO3+O2�T2H2SO4���������ԣ������������Ʒ�Ӧ������ʽΪH2SO3+2NaOH�TNa2SO3+2H2O���ʴ�Ϊ��
��4����ΪAl����ת����ϵ��֪NΪAl2O3��ZΪAl��OH��3��XΪAlCl3��YΪNH3��MΪNH4Cl��
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪAl3++3NH3?H2O�TAl��OH��3+3NH4+������NH4+����ȡ��������NaOH��Һ�����ȣ�����ʹʪ���ɫʯ����ֽ�����Ĵ̼������壬
���M�к���Ԫ��Cl��Ϊ�Ȼ�泥�Ϊǿ�������Σ�ˮ������ԣ���Һ������Ũ���ɴ�С������˳����C��Cl-����C��NH+4����C��H+����C��OH-����
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3+3NH4+��ȡ��������NaOH��Һ�����ȣ�����ʹʪ���ɫʯ����ֽ�����Ĵ̼������壻C��Cl-����C��NH+4����C��H+����C��OH-����
��1���ɢܡ��ݡ���Ԫ�����γɵļȺ����Ӽ��ֺ����ۼ���һ�����ӻ�����ΪNaClO������ʽΪ
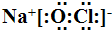 ���ʴ�Ϊ��
���ʴ�Ϊ��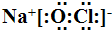 ��
����2���ɴ�������������ˮ������������Ӧ���������ԣ�A��ClԪ��Ϊ+1�ۣ���ˮ������HClO��C��D������ǿ�����ԣ�������ɱ���������ʴ�Ϊ��ACD��
��3��W��������ڵ�ͬ����Ԫ���к����ᣬ��ΪS����Ӧ��H2SO3���������ԡ���ԭ�Ժ����ԣ��ɱ���������H2SO4������Ϊ��ԭ�ԣ�����ʽΪ2H2SO3+O2�T2H2SO4���������ԣ������������Ʒ�Ӧ������ʽΪH2SO3+2NaOH�TNa2SO3+2H2O���ʴ�Ϊ��
| 1 | ��ԭ�� | 2H2SO3+O2�T2H2SO4 |
| 2 | ���� | H2SO3+2NaOH�TNa2SO3+2H2O |
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪAl3++3NH3?H2O�TAl��OH��3+3NH4+������NH4+����ȡ��������NaOH��Һ�����ȣ�����ʹʪ���ɫʯ����ֽ�����Ĵ̼������壬
���M�к���Ԫ��Cl��Ϊ�Ȼ�泥�Ϊǿ�������Σ�ˮ������ԣ���Һ������Ũ���ɴ�С������˳����C��Cl-����C��NH+4����C��H+����C��OH-����
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3+3NH4+��ȡ��������NaOH��Һ�����ȣ�����ʹʪ���ɫʯ����ֽ�����Ĵ̼������壻C��Cl-����C��NH+4����C��H+����C��OH-����
���������⿼���Ϊ�ۺϣ��漰Ԫ�����ڱ���������ƶϣ�Ϊ�߿��������ͣ�������ѧ���ķ���������Ԫ�ػ�����֪ʶ���ۺ����ã��Ѷ��еȣ�ע��������ʵ����ʣ�ѧϰ��ע����ۣ�

��ϰ��ϵ�д�
 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
�����Ŀ
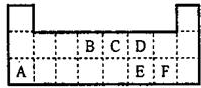 ��ͼΪԪ�����ڱ���һ���֣��������е���ĸ�ֱ����ijһԪ�أ�
��ͼΪԪ�����ڱ���һ���֣��������е���ĸ�ֱ����ijһԪ�أ�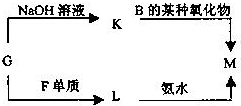
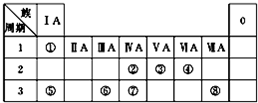 ��ͼΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
��ͼΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺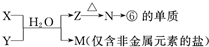
 ���Ŀռ�������Ϊ
���Ŀռ�������Ϊ