��Ŀ����
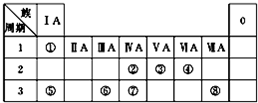 ��ͼΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
��ͼΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺��1��д���ɢܡ��ݡ���Ԫ�����γɵļȺ����Ӽ��ֺ����ۼ���һ�����ӻ�����ĵ���ʽ��
��2����Ԫ�آ�ĵ�����������ˮ���л��ﷴӦ���ɶ�������Σ�������ʣ���������̭��
���пɴ�����������ˮ����������
A��NH4Cl B��AlCl3 C��K2FeO4 D��ClO2
��3��W��������ڵ�ͬ����Ԫ�أ����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | ������ | H2WO3+3H3PO3�T3H3PO4+H2W�� |
| �� | ���� | |
| �� | ��ԭ�� |
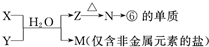
д��X��Һ��Y��Һ��Ӧ�����ӷ���ʽ
��������Ԫ�������ڱ��е�λ�ÿ�֪����ΪHԪ�ء���ΪCԪ�ء���ΪNԪ�ء���ΪOԪ�ء���ΪNaԪ�ء���ΪAlԪ�ء���ΪSiԪ�ء���ΪSԪ�أ�
��1����O��Na��Cl�γɵļȺ����Ӽ��ֺ����ۼ�������ΪNaClO�ȣ�NaClO�������������������ӹ��ɣ�
��2����Ԫ�آ�ĵ���Ϊ����������ǿ�����ԣ�����ϸ����������ѡ���о���ǿ�����Ե����ʿ������������
��3��W��������ڵ�ͬ����Ԫ�أ���ͼ��֪��������Ԫ�أ���WΪ��Ԫ�أ�H2SO3�������ԡ���ԭ�ԡ������ԣ���
��4��M�ǽ����ǽ�����������һ������Σ���ΪAlԪ�أ������ƶ�N������������Z���������������������Ϸ�Ӧ��X+Y+H2O��Al��OH��3+NH4+ ��֪���÷�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ���ݴ˽��
��1����O��Na��Cl�γɵļȺ����Ӽ��ֺ����ۼ�������ΪNaClO�ȣ�NaClO�������������������ӹ��ɣ�
��2����Ԫ�آ�ĵ���Ϊ����������ǿ�����ԣ�����ϸ����������ѡ���о���ǿ�����Ե����ʿ������������
��3��W��������ڵ�ͬ����Ԫ�أ���ͼ��֪��������Ԫ�أ���WΪ��Ԫ�أ�H2SO3�������ԡ���ԭ�ԡ������ԣ���
��4��M�ǽ����ǽ�����������һ������Σ���ΪAlԪ�أ������ƶ�N������������Z���������������������Ϸ�Ӧ��X+Y+H2O��Al��OH��3+NH4+ ��֪���÷�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ���ݴ˽��
����⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪHԪ�ء���ΪCԪ�ء���ΪNԪ�ء���ΪOԪ�ء���ΪNaԪ�ء���ΪAlԪ�ء���ΪSiԪ�ء���ΪSԪ�أ�
��1����O��Na��Cl�γɵļȺ����Ӽ��ֺ����ۼ�������ΪNaClO�ȣ�NaClO�������������������ӹ��ɣ���NaClO�ĵ���ʽΪ��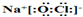 ��
��
�ʴ�Ϊ��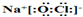 ��
��
��2����Ԫ�آ�ĵ���Ϊ����������ǿ�����ԣ�����ϸ����������ѡ���о���ǿ�����Ե����ʿ������������ѡ����K2FeO4��ClO2����ǿ�����ԣ�
�����CD��
��3��W��������ڵ�ͬ����Ԫ�أ���WΪSԪ�أ�H2SO3�ľ��������ԡ���ԭ�ԡ����ԡ����ȶ��Եȣ����Ա�ǿ��������������H2SO3+Br2+2H2O=H2SO3+2HBr����NaOH�����кͷ�ӦH2SO3+2NaOH=Na2SO3+2H2O��
�ʴ�Ϊ��
��4����6��M�ǽ����ǽ�����������һ������Σ���ΪAlԪ�أ������ƶ�N������������Z���������������������Ϸ�Ӧ��X+Y+H2O��Al��OH��3+NH4+ ��֪���÷�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ����
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ��Al3++3NH3+3H2O�TAl��OH��3��+3NH4+��
M�������ӵļ�������Ϊ��ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
�ʴ�Ϊ��Al3++3NH3+3H2O�TAl��OH��3��+3NH4+��ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
��1����O��Na��Cl�γɵļȺ����Ӽ��ֺ����ۼ�������ΪNaClO�ȣ�NaClO�������������������ӹ��ɣ���NaClO�ĵ���ʽΪ��
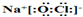 ��
���ʴ�Ϊ��
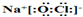 ��
����2����Ԫ�آ�ĵ���Ϊ����������ǿ�����ԣ�����ϸ����������ѡ���о���ǿ�����Ե����ʿ������������ѡ����K2FeO4��ClO2����ǿ�����ԣ�
�����CD��
��3��W��������ڵ�ͬ����Ԫ�أ���WΪSԪ�أ�H2SO3�ľ��������ԡ���ԭ�ԡ����ԡ����ȶ��Եȣ����Ա�ǿ��������������H2SO3+Br2+2H2O=H2SO3+2HBr����NaOH�����кͷ�ӦH2SO3+2NaOH=Na2SO3+2H2O��
�ʴ�Ϊ��
| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | H2WO3+3H3PO3�T3H3PO4+H2W�� | |
| 1 | ��ԭ�� | H2SO3+Br2+2H2O�TH2SO3+2HBr |
| 2 | ���� | H2SO3+2NaOH�TNa2SO3+2H2O |
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ��Al3++3NH3+3H2O�TAl��OH��3��+3NH4+��
M�������ӵļ�������Ϊ��ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
�ʴ�Ϊ��Al3++3NH3+3H2O�TAl��OH��3��+3NH4+��ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
���������⿼��Ԫ�ؼ�������ƶϡ����û�ѧ���Ԫ�ػ��������ʡ�ʵ�鷽����Ƶȣ�Ԫ�ؼ����ʵ��ƶ��ǽ����Ĺؼ���ע�ضԸ߿���������Ŀ��飬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
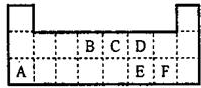 ��ͼΪԪ�����ڱ���һ���֣��������е���ĸ�ֱ����ijһԪ�أ�
��ͼΪԪ�����ڱ���һ���֣��������е���ĸ�ֱ����ijһԪ�أ�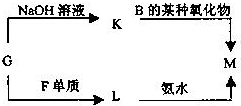

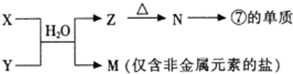
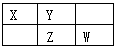
 ��ͼΪԪ�����ڱ���һ���֣�X��Y��Z��W��Ϊ������Ԫ�أ���Wԭ�ӵ��������������������������
��ͼΪԪ�����ڱ���һ���֣�X��Y��Z��W��Ϊ������Ԫ�أ���Wԭ�ӵ��������������������������