��Ŀ����
2����һƿ��ɫ���壬ȡ0.271g�����Թ��м��ȣ���������ֱ����ȫ��ʧ���Թܿ��д���ˮ�����ɣ�ȡ��ͬ�������ֹ���������ļ�ʯ�ҹ��ȣ�����һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬��Щ����ǡ���ܺ�25ml0.1mol/L��������Һ��ȫ��Ӧ����ȡͬ���������Ĺ����������ϡ�������ã����ɵ�����ȫ��ͨ��ʯ��ˮ�У���0.30g��ɫ��������1���ð�ɫ���������������Ӻ������ӵ����ʵ���֮���Ƕ��٣�
��2����ƿ��ɫ��������Щ������ɣ�0.271g��Ʒ�и���ֵ����ʵ����ֱ��Ƕ��٣�
���� ��ʹʪ���ɫʯ����ֽ����������Ϊ������������������ʵ��������0.271g�ù����к���笠����ӵ����ʵ����������ᷴӦ���ɵ���ɫ����ζ���ܹ�ʹ����ʯ��ˮ����ǵ�����Ϊ������̼�����ɵİ�ɫ����Ϊ̼��ƣ�����̼��Ƶ����ʵ������Լ������ɫ�����к���̼�������̼��������ӵ����ʵ��������ݼ��ȹ������ȫ�ֽ���û�в����˵���ð�ɫ����Ϊ̼����炙�̼��炙����ǵĻ����������ɵİ����Ͷ�����̼�����ʵ����ɼ����笠����ӡ�̼�����̼��������ӵ����ʵ���������ȷ����ɫ�������ɣ�
��� �⣺��1���������Һ����ʱ�ų���ʹʪ���ɫʯ����ֽ���������壬֤����NH4+���ڣ���Щ����ǡ���ܺ�25ml0.1mol/L��������Һ��ȫ��Ӧ����n��NH4+��=2��n��H2SO4��=2��0.100mol/L��0.025L=0.005mol���������ᷴӦ�����ɵ���ɫ����������ʯ��ˮ���ò�����ɫ������֤ʵ��������CO32-��HCO3-��
n��CO32-��HCO3-��=n��CaCO3��=$\frac{0.300g}{100g/mol}$=0.003mol���ù����������������ʵ���֮��Ϊ��0.003mol��0.005mol=3��5��
�𣺸ù����������������ʵ���֮��Ϊ3��5��
��2���ù�����ȷֽ���Թ���û���κβ�����Թܿ��д���ˮ�����ɣ��Լ����ɳ�ˮ����֮��ֻ���������壬˵���ù����в����κν������Ӻ�����������ӣ����ݣ�1������õ������������ʵ���֮�ȿ�֪���ù���Ϊ��NH4��2CO3��NH4HCO3�Ļ���
�裨NH4��2CO3�����ʵ���Ϊx��NH4HCO3�����ʵ���Ϊy������笠����Ӻ�Cԭ���غ�ã�
$\left\{\begin{array}{l}{x+y=0.003mol}\\{2x+y=0.005mol}\end{array}\right.$����ã�$\left\{\begin{array}{l}{x=0.002mol}\\{y=0.001mol}\end{array}\right.$�����������Ʒ��������0.002��96g+0.001��79g=0.271g�����ϲ�˵���ù���Ϊ��NH4��2CO3��NH4HCO3�Ļ����
����ƿ��ɫ�����ɣ�NH4��2CO3��NH4HCO3��ɣ�0.271g��Ʒ�и�����У���NH4��2CO3�����ʵ���Ϊ0.002mol��NH4HCO3�����ʵ���Ϊ0.001mol��
���� ���⿼����δ֪����飬�ж���������ϣ���ѧ�����ۺ�����Ҫ����ߣ�Ҫע��Ի���֪ʶ�����ϣ�ͬʱ��Ҫ���ڽ���ֽ�����ɸ�С�⣬�������ƣ�Ȼ�����ۺϿ��ǣ�

| A�� | ����ƿ����Һ©�����ζ�����ʹ�ø�ǰ����Ҫ�����Ƿ�©ˮ����������ͬ�ķ�������Ƿ�©ˮ | |
| B�� | ��Һ��������ʱ��������Һ��������ܳ����ݻ���1/2 | |
| C�� | ǿ��ζ�ǿ��ʱ���÷�ָ̪ʾ�����ü��ȸ����жϵζ��յ� | |
| D�� | ���ʵķ����ᴿ����֮һΪ��ɸ�֡����罺��һ��Ĥ�������ᴿ������Һһ���˷��룬�����Ͼ���������������ӵ�ֱ����Сѡ����к��ʿ��ġ�ɸ�ӡ� |
| A�� | ��μ��백ˮ�У�������Һ��c��H+�������� | |
| B�� | ����ͨ�����C12���١��ھ������ƶ�����ҺpH��С | |
| C�� | ����CaCO3����Һ�У�CaCO3���ܽ�ƽ�������ƶ� | |
| D�� | ����һ����NaOH��Һ�У�������Һ������Ũ�ȵĹ�ϵ����Ϊc��Cl-��+c��ClO-��=c��Na+�� |
| A�� | ��֪��O�TO������Ϊa kJ/mol��H-H������Ϊb kJ/mol��ˮ������H-O����Ϊc kJ/mol����֪��H2O��g���TH2O��l����H=-d kJ/mol����Ӧ2H2 ��g��+O2��g���T2H2O��l�� �ġ�HΪ����a+2b-4c-2d��kJ/mol | |
| B�� | Ϊȷ��ij��H2A��ǿ�ỹ�����ᣬ�ɲⳣ����NaHA��Һ��pH����������pH��7����H2A�������pH��7����H2A��ǿ�� | |
| C�� | ������pH��ͬ��CH3COONa��Һ��C6H5ONa��Һ��Na2CO3��Һ��NaOH��Һ������ҺŨ�ȴ�С��ϵ��c��CH3COONa����c��Na2CO3����c��C6H5ONa����c��NaOH�� | |
| D�� | ��ͬ�¶��£��������Ȼ�������ֱ������ͬ����Ģ�����ˮ����0.1 mol/L���ᡢ��0.1 mol/L�Ȼ�þ��Һ����0.1 mol/L��������Һ�У�Ag+Ũ�ȣ��٣���=�ڣ��� |
| A�� | 1 mol CH3+����̼�����ӣ����еĵ�����ĿΪ9NA | |
| B�� | 7.2g C5H12���е�C-C������ĿΪ0.5NA | |
| C�� | ���³�ѹ�����£�14 g N2��CO�Ļ�����庬�еķ�����ĿΪ0.5NA | |
| D�� | 2mol H2O2��ȫ�ֽ�����1mol O2��ת�Ƶĵ�����Ϊ4NA |
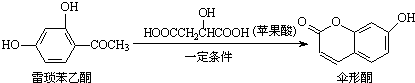
�����й����������ʵ�˵����ȷ���ǣ�������
| A�� | ��������ͪ����̼ԭ�Ӳ����ܹ�ƽ�� | |
| B�� | ��������ͪ��ɡ��ͪ���ܸ�FeCl3��Һ������ɫ��Ӧ | |
| C�� | 1 mol��������ͪ������H2��Ӧ���������3 mol H2 | |
| D�� | 1 molɡ��ͪ������NaOH��Һ��Ӧ���������3 mol NaOH |
| A�� | �ڵĵ����ڳ������ǹ�̬ | B�� | �ڵij������ϼ���-2��+4��+6 | ||
| C�� | �ڿ������뵼����� | D�� | �ڵ��⻯��H2Te���ȶ� |
| A�� | ��ɫ���ӷ����д̼�����ζ��Һ�� | |
| B�� | Ũ��Ϊ98%���ϵ�Ũ����з������ᣬ������ˮ�� | |
| C�� | Ũ����ͨ���Ի�ɫ����Ϊ�ܽ��������Ķ������� | |
| D�� | ����ȶ���Ũ��Խ��Խ�ֽ� |
 ��
��