��Ŀ����
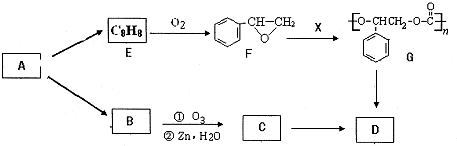
��֪A��G����ͼ��ʾ��ת����ϵ����������������ȥ��������A��GΪ���ʣ�D����ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬E��F������NaOH��Һ��Ӧ��
��ش��������⣺
��1��д��F�ĵ���ʽ�� ��
��2����C��Һ��D��Ӧ�����ӷ���ʽΪ ��
��F��Һ��NaOH��Һ���ȷ�Ӧ�Ļ�ѧ����ʽΪ ��
��3����C��Һ�� ������ԡ��������ԡ����ԡ����������� �������ӷ���ʽ���ͣ�
��F��Һ������Ũ���ɴ�С��˳��Ϊ ��
��4����5.4g AͶ��200mL 2.0mol/Lij��Һ����G���ʲ������ҳ�ַ�Ӧ���н���ʣ�࣬�����Һ������ ������ţ���
A��HNO3��Һ B��H2SO4��Һ C��NaOH��Һ D��HCl��Һ
��5��A����B�Ļ�ѧ��Ӧ����ʽΪ ��д��Ӧ�ø÷�Ӧԭ����һ����;�� ��

��ش��������⣺
��1��д��F�ĵ���ʽ��
��2����C��Һ��D��Ӧ�����ӷ���ʽΪ
��F��Һ��NaOH��Һ���ȷ�Ӧ�Ļ�ѧ����ʽΪ
��3����C��Һ��
��F��Һ������Ũ���ɴ�С��˳��Ϊ
��4����5.4g AͶ��200mL 2.0mol/Lij��Һ����G���ʲ������ҳ�ַ�Ӧ���н���ʣ�࣬�����Һ������
A��HNO3��Һ B��H2SO4��Һ C��NaOH��Һ D��HCl��Һ
��5��A����B�Ļ�ѧ��Ӧ����ʽΪ
���㣺������ƶ�
ר�⣺�ƶ���
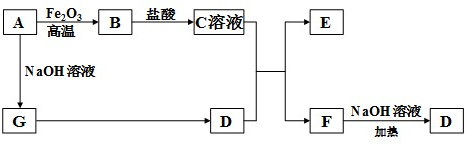
������A��G����ͼ��ʾ��ת����ϵ����������������ȥ����D����ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬��DΪNH3������A��GΪ���ʣ���A����������Ӧ�����������Ʒ�Ӧ��Ӧ�õ�G��G��Ӧ���Եõ�����������֪AΪAl��GΪH2��E��F������NaOH��Һ��Ӧ�����ת����ϵ��֪����BΪAl2O3��CΪAlCl3��EΪAl��OH��3��FΪNH4Cl���ݴ˽��
���
�⣺A��G����ͼ��ʾ��ת����ϵ����������������ȥ����D����ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬��DΪNH3������A��GΪ���ʣ���A����������Ӧ�����������Ʒ�Ӧ��Ӧ�õ�G��G��Ӧ���Եõ�����������֪AΪAl��GΪH2��E��F������NaOH��Һ��Ӧ�����ת����ϵ��֪����BΪAl2O3��CΪAlCl3��EΪAl��OH��3��FΪNH4Cl��
��1��FΪNH4Cl������ʽΪ��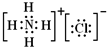 ���ʴ�Ϊ��
���ʴ�Ϊ��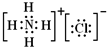 ��
��
��2����C��Һ��D��Ӧ�����ӷ���ʽΪ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
�ʴ�Ϊ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
��F��Һ��NaOH��Һ���ȷ�Ӧ�Ļ�ѧ����ʽΪ��NH4Cl+NaOH
NaCl+NH3��+H2O��
�ʴ�Ϊ��NH4Cl+NaOH
NaCl+NH3��+H2O��
��3����AlCl3��Һ��������ˮ�⣺Al3++3H2O?Al��OH��3+3H+��ƽ��ˮ�ĵ���ƽ�⣬��Һ�����ԣ�
�ʴ�Ϊ���Al3++3H2O?Al��OH��3+3H+��
��NH4Cl��Һ��笠�����ˮ�⣬��Һ�����ԣ�����Һ������Ũ���ɴ�С��˳��Ϊ��c��Cl-����c��NH4+����c��H+����c��OH-����
�ʴ�Ϊ��c��Cl-����c��NH4+����c��H+����c��OH-����
��4����5.4g Al�����ʵ���=
=0.2mol��200mL 2.0mol/Lij��Һ�����ʵ����ʵ���=0.2L��2mol/L=0.4mol����AlͶ�����Һ����G���ʲ������ҳ�ַ�Ӧ���н���ʣ�࣬Al��ϡ���ᷴӦû�����嵥�����ɣ���ϡ���ᡢ�������ơ����ᷴӦ������������
��2Al��3H2SO4��֪��0.2molAl��ȫ��Ӧ����H2SO4 �����ʵ���=0.3mol��0.4mol����Alû��ʣ�࣬
��2Al��2NaOH��֪��0.2molAl��ȫ��Ӧ����NaOH�����ʵ���=0.2mol��0.4mol����Alû��ʣ�࣬
��2Al��6HCl��֪��0.2molAl��ȫ��Ӧ����HCl�����ʵ���=0.6mol��0.4mol����Al��ʣ�࣬
�ʴ�Ϊ��D��
��5��A����B�Ļ�ѧ��Ӧ����ʽΪ��2Al+Fe2O3
Al2O3+2Fe���÷�Ӧԭ����;�У����Ӹֹ졢��ҵ��ұ�����۵Ľ����ȣ�
�ʴ�Ϊ��2Al+Fe2O3
Al2O3+2Fe�����Ӹֹ��ұ�����۵Ľ����ȣ�
��1��FΪNH4Cl������ʽΪ��
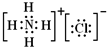 ���ʴ�Ϊ��
���ʴ�Ϊ��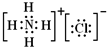 ��
����2����C��Һ��D��Ӧ�����ӷ���ʽΪ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
�ʴ�Ϊ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
��F��Һ��NaOH��Һ���ȷ�Ӧ�Ļ�ѧ����ʽΪ��NH4Cl+NaOH
| ||
�ʴ�Ϊ��NH4Cl+NaOH
| ||
��3����AlCl3��Һ��������ˮ�⣺Al3++3H2O?Al��OH��3+3H+��ƽ��ˮ�ĵ���ƽ�⣬��Һ�����ԣ�
�ʴ�Ϊ���Al3++3H2O?Al��OH��3+3H+��
��NH4Cl��Һ��笠�����ˮ�⣬��Һ�����ԣ�����Һ������Ũ���ɴ�С��˳��Ϊ��c��Cl-����c��NH4+����c��H+����c��OH-����
�ʴ�Ϊ��c��Cl-����c��NH4+����c��H+����c��OH-����
��4����5.4g Al�����ʵ���=
| 5.4g |
| 27g/mol |
��2Al��3H2SO4��֪��0.2molAl��ȫ��Ӧ����H2SO4 �����ʵ���=0.3mol��0.4mol����Alû��ʣ�࣬
��2Al��2NaOH��֪��0.2molAl��ȫ��Ӧ����NaOH�����ʵ���=0.2mol��0.4mol����Alû��ʣ�࣬
��2Al��6HCl��֪��0.2molAl��ȫ��Ӧ����HCl�����ʵ���=0.6mol��0.4mol����Al��ʣ�࣬
�ʴ�Ϊ��D��
��5��A����B�Ļ�ѧ��Ӧ����ʽΪ��2Al+Fe2O3
| ||
�ʴ�Ϊ��2Al+Fe2O3
| ||
���������⿼�������ƶϣ�D���������ƶ�ͻ�ƿڣ��ٽ��ת����ϵ�����ⷴӦ�ƶϣ��Ѷ��еȣ�ע��Ԫ�ػ�����֪ʶ�����գ�

��ϰ��ϵ�д�
�����Ŀ
������������ķ�����һ����ȵ��ǣ�������
| A�������ȡ��������ȵ�CO��N2 |
| B�����µ������O2��N2 |
| C����������ܶȵ�CO��N2 |
| D����ѹ�������O2��N2 |
��ѧ��ѧ�������ʼס��ҡ�������֮�����ת����ϵ����ʮ�ҡ���ʮ��������˵����ȷ���ǣ�������
| A������Ϊ�Ƶ��ʣ���Ϊ����������һ����ˮ |
| B������Ϊ�����ʣ���Ϊ�����ʣ�����һ���������� |
| C������Ϊͭ���ʣ���Ϊ�Ȼ�������Һ������һ�����Ȼ�����Һ |
| D�����ס��ҡ���������Ϊ�������÷�Ӧһ�����ڸ��ֽⷴӦ |
���й�����֬�����������ıȽ��У���ȷ���ǣ�������
| A����֬�������������Ǵ�����������¶���Һ�� |
| B����֬��������������ˮ����������ʹ� |
| C����֬����������������ʹ��ˮ��ɫ |
| D����֬������������������ˮ�����������л��ܼ� |