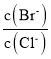��Ŀ����
����Ŀ�������������£���pH�������ͬ����������(HNO2��CH3COOH)�ֱ��ˮϡ�ͣ���pH���ˮ����ı仯����ͼ��ʾ��
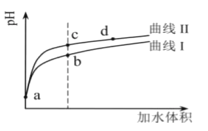
��֪���������£�HNO2��CH3COOH�ĵ��볣���ֱ�Ϊ5.0��10-4��1.7��10-5������˵����ȷ����
A.����II��������CH3COOH��Һ
B.��Һ��ˮ�ĵ���̶ȣ�b��>c��>d��
C.�����ͬ��a������Һ�ֱ���NaOH��Һǡ���к�ʱ��������Һ�У�n(![]() )��n(Na��)��n(CH3COO-)
)��n(Na��)��n(CH3COO-)
D.��c�㵽d�㣬��Һ��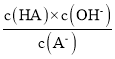 ���ֲ���(����HA��A-�ֱ������Ӧ������������)
���ֲ���(����HA��A-�ֱ������Ӧ������������)
���𰸡�D
��������
A����ĵ���ƽ�ⳣ��Խ���������Խǿ��pH��ͬ��HNO2��CH3COOH�ֱ��ˮϡ����ͬ�ı�����pHֵ�仯�ϴ����ǿ�ᣬ����ͼ֪������I��ʾCH3COOH������II��ʾHNO2����A����
B��������ˮ���룬����c(H+)Խ��������ˮ����̶�Խ������c(H+)��b��c��d����ˮ�ĵ���̶ȣ�b��c��d����B����
C����ͬ�����a��������Һ��n(��)��n(HNO2)��n(CH3COOH)���ֱ���NaOHǡ���кͺ�����n(NaOH)��HNO2��CH3COOH����Ӧ����Һ��n(NaNO2)��n(CH3COONa)����������Һ�У�n(![]() )��n(CH3COO-)��n(Na��)����C����
)��n(CH3COO-)��n(Na��)����C����
D���¶Ȳ���ˮ��ƽ�ⳣ�����䣬��c�㵽d���¶Ȳ��䣬��Һ��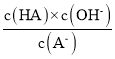 =Kh(HA)���䣬��D��ȷ��
=Kh(HA)���䣬��D��ȷ��
�ʴ�ΪD��

����Ŀ��ijѧϰС����0.80 mol/L��Ũ�ȵ��ռ���Һ�ⶨδ֪Ũ�ȵ����ᡣ
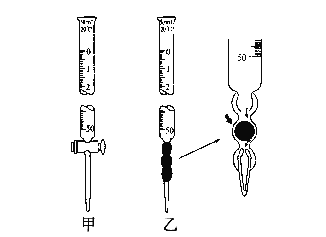
(1).�ζ�����ͼ��ʾ����______�ζ���ʢװ��Ũ�ȵ�����������Һ (����������������)��
(2).�õζ��ķ������ⶨ�����Ũ�ȣ�ʵ������������ʾ��
ʵ���� | ����HCl��Һ�����/mL | ����NaOH��Һ�����/mL |
1 | 20.00 | 23.00 |
2 | 20.00 | 23.10 |
3 | 20.00 | 22.90 |
��ʵ������ȥ��Һ�����Ϊ________ml��
(3).���в�����ʹ����õ������Ũ��ƫ�͵���________��
A��ʢװ����Һ����ƿ��ˮϴ��δ����
B���ζ�ǰ����ʽ�ζ��ܼ�������ݣ��ζ���������ʧ
C����ʽ�ζ���������ˮϴ����δ�ñ�����������Һ��ϴ
D������ʽ�ζ��ܵĿ̶�ʱ���ζ�ǰ���Ӱ�Һ����ʹ����ζ����Ӷ���
(4).�ζ������У��۾�Ӧע��________��������̨�ϵ�һ�Ű�ֽ����Ŀ����_______________��
���÷�̪��ָʾ�������жϴﵽ�ζ��յ�ʱ��������__________________��
(5).�����ϱ����ݣ����㱻����������ʵ���Ũ����________mol/L��(С���������λ��Ч����)