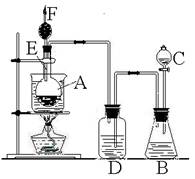
��Ŀ����
��һ��ʢ��Ũ������Լ�ƿ��ǩ��Ӧӡ�����о�ʾ����е�
��������ͼ�������Լ�ƿ��ǩ�ϵIJ������ݣ�

��ʵ������Ҫ240ml 0.46mol/L��ϡ�����ø��������ã���
��1����Ҫ��Ũ��������Ϊ
��2���ɹ�ѡ�õ������У���ͷ�ιܣ���ƿ���ձ���ҩ�ף���Ͳ��������ƽ������ϡ����ʱ����ȱ�ٵ�������
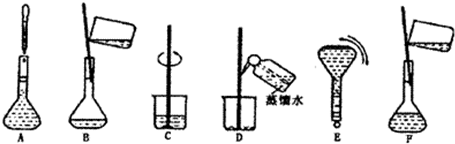
��3�����ù������м����ؼ��IJ���Ͳ�����ͼ��ʾ��������ʵ�鲽��A-F��ʵ������Ⱥ��������
��4����ͬѧʵ�����õõ���Ũ��Ϊ0.45mol/L�����ܵ�ԭ����
A����ȡŨH2SO4ʱ���ӿ̶�
B������ƿϴ����δ�����ﴦ��
C����ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�����
D������ʱ���ӿ̶��ߣ�
D
D
��
��������ͼ�������Լ�ƿ��ǩ�ϵIJ������ݣ�

��ʵ������Ҫ240ml 0.46mol/L��ϡ�����ø��������ã���
��1����Ҫ��Ũ��������Ϊ
6.3
6.3
mL����2���ɹ�ѡ�õ������У���ͷ�ιܣ���ƿ���ձ���ҩ�ף���Ͳ��������ƽ������ϡ����ʱ����ȱ�ٵ�������
������
������
��250mL����ƿ
250mL����ƿ
��3�����ù������м����ؼ��IJ���Ͳ�����ͼ��ʾ��������ʵ�鲽��A-F��ʵ������Ⱥ��������
CBDFAE
CBDFAE
��
��4����ͬѧʵ�����õõ���Ũ��Ϊ0.45mol/L�����ܵ�ԭ����
AD
AD
��A����ȡŨH2SO4ʱ���ӿ̶�
B������ƿϴ����δ�����ﴦ��
C����ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�����
D������ʱ���ӿ̶��ߣ�
��������һ������Ũ�������ڸ�ʴ�Ե�ҩƷ�����жϣ�
��������1����Ҫ240ml 0.46mol/L��ϡ���ᣬʵ������ʱ��Ҫ����250mlϡ������Һ��
��2����������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ѡ��������
��3����������һ�����ʵ���Ũ�ȵ���Һ�IJ����������
��4�����Ƶ�Ũ��ƫС�����ݲ�����c=
��Ӱ������жϣ�
��������1����Ҫ240ml 0.46mol/L��ϡ���ᣬʵ������ʱ��Ҫ����250mlϡ������Һ��
��2����������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ѡ��������
��3����������һ�����ʵ���Ũ�ȵ���Һ�IJ����������
��4�����Ƶ�Ũ��ƫС�����ݲ�����c=
| n |
| V |
����⣺��һ��Ũ������к�ǿ�ĸ�ʴ�ԣ�ʢ��Ũ������Լ�ƿ��ǩ��Ӧӡ�о��и�ʴ�Եı�־������D��ȷ��
�ʴ�Ϊ��D��
���� ����1����Ũ�����к��е���������ʵ���Ũ��Ϊ��
=18.4mol/L��������Ҫ240ml 0.46mol/L��ϡ���ᣬʵ������ʱ��Ҫ����250mL��ϡ���ᣬ
��Ҫ��������ʵ���Ϊ��0.46mol/L��0.25L=0.115mol����ҪŨ�������Ϊ��
=0.00625L=6.25mL��������Ͳֻ��ȷ��0.1mL����������Ͳ��ȡ��Ũ�������Ϊ6.3mL��
�ʴ�Ϊ��6.3��
��2������һ�����ʵ���Ũ�ȵ���Һ�IJ���Ϊ�����㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݵȣ���Ҫ�������У���Ͳ���ձ�����ͷ�ιܡ���������250mL����ƿ�����Ի���Ҫ��������250mL����ƿ��
�ʴ�Ϊ����������250ml������ƿ��
��3������һ�����ʵ���Ũ�ȵ���Һ�IJ����У����㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�������ȷ˳��Ϊ��CBDFAE��
�ʴ�Ϊ��CBDFAE��
��4����ͬѧʵ�����õõ���Ũ��Ϊ0.45mol/L��˵�����Ƶ���Һ��Ũ��ƫ�ͣ�
A����ȡŨH2SO4ʱ���ӿ̶ȣ�������ȡ��Ũ��������ƫС��������������ʵ���Ũ��ƫ�ͣ���A��ȷ��
B������ƿϴ����δ�����ﴦ���������ƽ���������Ӱ�죬��B����
C����ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�������û����ȴ��������ȴ�����Ƶ���Һ�����ƫС����Һ��Ũ��ƫ��C����
D������ʱ���ӿ̶��ߣ��������Ƶ���Һ�����ƫ����c=
���������Ƶ���Һ��Ũ��ƫС����D��ȷ��
�ʴ�Ϊ��AD��
�ʴ�Ϊ��D��
���� ����1����Ũ�����к��е���������ʵ���Ũ��Ϊ��
| ||
| 1L |
��Ҫ��������ʵ���Ϊ��0.46mol/L��0.25L=0.115mol����ҪŨ�������Ϊ��
| 0.115mol |
| 18.4mol/L |
�ʴ�Ϊ��6.3��
��2������һ�����ʵ���Ũ�ȵ���Һ�IJ���Ϊ�����㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݵȣ���Ҫ�������У���Ͳ���ձ�����ͷ�ιܡ���������250mL����ƿ�����Ի���Ҫ��������250mL����ƿ��
�ʴ�Ϊ����������250ml������ƿ��
��3������һ�����ʵ���Ũ�ȵ���Һ�IJ����У����㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�������ȷ˳��Ϊ��CBDFAE��
�ʴ�Ϊ��CBDFAE��
��4����ͬѧʵ�����õõ���Ũ��Ϊ0.45mol/L��˵�����Ƶ���Һ��Ũ��ƫ�ͣ�
A����ȡŨH2SO4ʱ���ӿ̶ȣ�������ȡ��Ũ��������ƫС��������������ʵ���Ũ��ƫ�ͣ���A��ȷ��
B������ƿϴ����δ�����ﴦ���������ƽ���������Ӱ�죬��B����
C����ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�������û����ȴ��������ȴ�����Ƶ���Һ�����ƫС����Һ��Ũ��ƫ��C����
D������ʱ���ӿ̶��ߣ��������Ƶ���Һ�����ƫ����c=
| n |
| V |
�ʴ�Ϊ��AD��
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ��������������漰��������Һʱ������ѡ�����ʵ����Ѷȵļ��㼰�����������Ը�����ѧ֪ʶ��ɣ������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
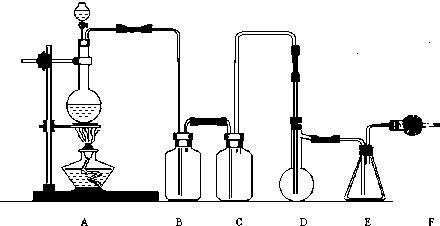
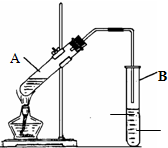 ʵ����������ͼ��ʾװ����ȡ������������ش�
ʵ����������ͼ��ʾװ����ȡ������������ش�