��Ŀ����
6���о��������Ի�����MΪԭ�Ϻϳɵ���ζ��ͪ�����ĺ�Ĭ֢��������մ�֢���������õ�����Ч����M��һ�ֺϳ�·����ͼ��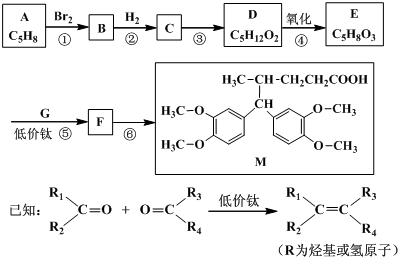
�ش��������⣺
��1��A�Ľṹ��ʽ��CH2=CHCH=CHCH3��A��һ��ͬ���칹�����γ���Ȼ�ĵ��壬��������2-��-1��3-����ϩ���������ϩ����
��2��G�ķ���ʽ��C17H18O5����Ӧ�ķ�Ӧ�����Ǽӳɣ���ԭ����
��3����Ӧ��������������O2������������1mol D������1.5molO2��д����Ӧ�ٵĻ�ѧ����ʽCH2=CHCH=CHCH3+Br2��CH2BrCH=CHCHBrCH3��
��4����Ӧ��������F��ͬʱ���γ����ָ����д��ʽ����С�ĸ�����Ľṹ��ʽHOOCCH2CH2C��CH3��=C��CH3��CH2CH2COOH��
��5��C��ijͬ���칹����ֻ�������ֻ�ѧ������ͬ����ԭ�ӣ�����ܵĽṹ��3�֣���д��һ�ֵĽṹ��ʽ
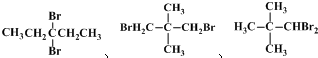 ������֮һ������
������֮һ��������6����EΪԭ�ϣ���ѡ���Լ��ɺϳɻ�ѧʽΪ��C5H8O2��n�ĸ߾��д����ط�Ӧ�Ļ�ѧ����ʽ��
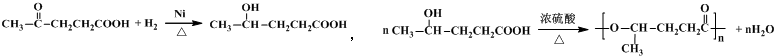 ��
��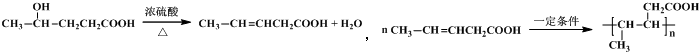 ��
��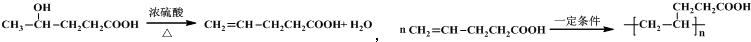 ��
��
���� ����A��D��E�ķ���ʽ�ȽϿ�֪��A�в����Ͷ�Ϊ2��DΪ���͵Ķ�Ԫ����D������E����EӦ����һ���ʻ���һ���Ȼ�������M�Ľṹ��ʽ��������Ϣ��֪��E��G������Ϣ�еķ�Ӧ����F��F�������ӳɵ�M������FΪ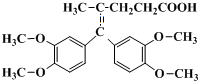 ��GΪ
��GΪ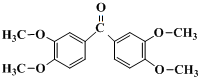 ��EΪHOOCCH2CH2COCH3������E�Ľṹ����֪AΪCH2=CHCH=CHCH3��A���巢��1��4�ӳɵ�BΪCH2BrCH=CHCHBrCH3��B�����������ӳɷ�Ӧ��CΪCH2BrCH2CH2CHBrCH3��C����ˮ���DΪCH2OHCH2CH2CHOHCH3���ݴ˴��⣮
��EΪHOOCCH2CH2COCH3������E�Ľṹ����֪AΪCH2=CHCH=CHCH3��A���巢��1��4�ӳɵ�BΪCH2BrCH=CHCHBrCH3��B�����������ӳɷ�Ӧ��CΪCH2BrCH2CH2CHBrCH3��C����ˮ���DΪCH2OHCH2CH2CHOHCH3���ݴ˴��⣮
��� �⣺����A��D��E�ķ���ʽ�ȽϿ�֪��A�в����Ͷ�Ϊ2��DΪ���͵Ķ�Ԫ����D������E����EӦ����һ���ʻ���һ���Ȼ�������M�Ľṹ��ʽ��������Ϣ��֪��E��G������Ϣ�еķ�Ӧ����F��F�������ӳɵ�M������FΪ ��GΪ
��GΪ ��EΪHOOCCH2CH2COCH3������E�Ľṹ����֪AΪCH2=CHCH=CHCH3��A���巢��1��4�ӳɵ�BΪCH2BrCH=CHCHBrCH3��B�����������ӳɷ�Ӧ��CΪCH2BrCH2CH2CHBrCH3��C����ˮ���DΪCH2OHCH2CH2CHOHCH3��
��EΪHOOCCH2CH2COCH3������E�Ľṹ����֪AΪCH2=CHCH=CHCH3��A���巢��1��4�ӳɵ�BΪCH2BrCH=CHCHBrCH3��B�����������ӳɷ�Ӧ��CΪCH2BrCH2CH2CHBrCH3��C����ˮ���DΪCH2OHCH2CH2CHOHCH3��
��1����������ķ�����֪��A�Ľṹ��ʽ�� CH2=CHCH=CHCH3��A��һ��ͬ���칹�����γ���Ȼ�ĵ��壬��������2-��-1��3-����ϩ���������ϩ����
�ʴ�Ϊ��CH2=CHCH=CHCH3��2-��-1��3-����ϩ���������ϩ����
��2��GΪ ��G�ķ���ʽ�� C17H18O5����Ӧ��Ϊ�ӳɣ���ԭ����Ӧ��
��G�ķ���ʽ�� C17H18O5����Ӧ��Ϊ�ӳɣ���ԭ����Ӧ��
�ʴ�Ϊ��C17H18O5���ӳɣ���ԭ����
��3����1molCH2OHCH2CH2CHOHCH3��O2����������������HOOCCH2CH2COCH3��Ҫ���� 1.5molO2����Ӧ�ٵĻ�ѧ����ʽΪCH2=CHCH=CHCH3+Br2��CH2BrCH=CHCHBrCH3��
�ʴ�Ϊ��1.5��CH2=CHCH=CHCH3+Br2��CH2BrCH=CHCHBrCH3��
��4����Ӧ��������F��ͬʱ���γ����ָ����ʽ����С�ĸ�����Ϊ����HOOCCH2CH2COCH3����֮�䷢����Ϣ�еķ�Ӧ��������Ľṹ��ʽΪHOOCCH2CH2C��CH3��=C��CH3��CH2CH2COOH��
�ʴ�Ϊ��HOOCCH2CH2C��CH3��=C��CH3��CH2CH2COOH��
��5��CΪCH2BrCH2CH2CHBrCH3��C��ijͬ���칹����ֻ�������ֻ�ѧ������ͬ����ԭ�ӣ�����ܵĽṹΪ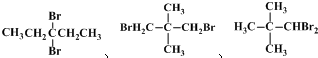 ������3�֣�
������3�֣�
�ʴ�Ϊ��3��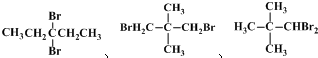 ������֮һ����
������֮һ����
��6��EΪHOOCCH2CH2COCH3����EΪԭ�ϣ���ѡ���Լ��ɺϳɻ�ѧʽΪ��C5H8O2��n�ĸ߾��������E�����������ӳɷ�Ӧ���ٷ������۷�Ӧ����Ӧ�Ļ�ѧ����ʽΪ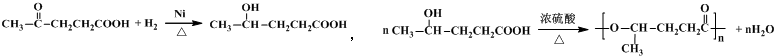 ��
��
����E�������ӳɺ�IJ������ȥ��Ӧ����̼̼˫�����ٷ����Ӿ۷�Ӧ����Ӧ�ķ���ʽΪ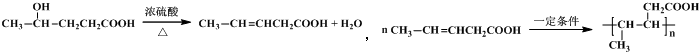 ��
��
����E�������ӳɺ�IJ�������۷�Ӧ����Ӧ�ķ���Ϊ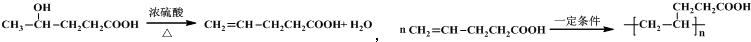 ��
��
�ʴ�Ϊ��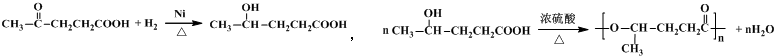 ��
��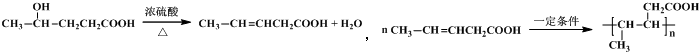 ��
��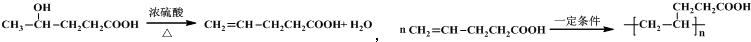 ��
��
���� �������л���ϳɿ����л���Ľṹ�����ʣ���Ŀ�ѶȽϴ���ע��������ʵĽṹ�����ƶϣ�ע���л������ŵ��ת����

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д� �����������ǣ�������
�����������ǣ�������| A�� | 3-������ | B�� | 2��3-�������� | C�� | 2-������ | D�� | 3-������ |
| A�� | MgO | B�� | NaOH | C�� | H2SO4 | D�� | BaCO3 |
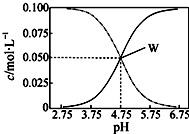 25�棬��c��CH3COOH��+c��CH3COO-��=0.1mol/L��һ�����ʹ����ƻ����Һ����Һ��c��CH3COOH����c��CH3COO-����pHֵ�Ĺ�ϵ��ͼ��ʾ���й�����Ũ�ȹ�ϵ������ȷ���ǣ�������
25�棬��c��CH3COOH��+c��CH3COO-��=0.1mol/L��һ�����ʹ����ƻ����Һ����Һ��c��CH3COOH����c��CH3COO-����pHֵ�Ĺ�ϵ��ͼ��ʾ���й�����Ũ�ȹ�ϵ������ȷ���ǣ�������| A�� | pH=5.5��Һ�У�c��CH3COO-����c��CH3COOH����c��H+����c��OH-�� | |
| B�� | W���ʾ��Һ�У�c��Na+��+c��H+��=c��CH3COO-��+c��CH3COOH�� | |
| C�� | pH=3.5��Һ�У�c��Na+��-c��OH-��+c��CH3COOH��=0.1 mol/L | |
| D�� | ��W������ʾ��Һ��ͨ��0.05molHCl���壨��Һ����仯�ɺ��ԣ���c��H+��=c��CH3COOH��+c��OH-�� |
 ʵ���ҳ����õ�ΰ�����ɲ���ʵ�飬�����ȿ��Խ�ԼҩƷ���������ֱ��ڹ۲�ʵ������ͼ����ʾ��ʵ�飬��ΰ���������ʵ��������ȷ���ǣ�������
ʵ���ҳ����õ�ΰ�����ɲ���ʵ�飬�����ȿ��Խ�ԼҩƷ���������ֱ��ڹ۲�ʵ������ͼ����ʾ��ʵ�飬��ΰ���������ʵ��������ȷ���ǣ���������װ��ͭƬ�Ŀ�Ѩ����Һ����ɫ����װ�е����Ŀ�Ѩ�й������ɫ��dz����װ����Ƭ�Ŀ�Ѩ���к���ɫ�������ɣ���װ�е�����Һ�Ŀ�Ѩ�г�dz��ɫ��
| A�� | �٢� | B�� | �ڢ� | C�� | �ڢ� | D�� | �٢� |
| A�� | �������� | B�� | ������ | C�� | ������ | D�� | ����ͭ |
| ѡ�� | ʵ �� �� �� �� �� �� | ʵ �� �� �� |
| A | ��ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ����Һ��һ������SO42- |
| B | ��ij��Һ�м���2��KSCN��Һ����Һ���Ժ�ɫ��������Һ�м��뼸�����Ƶ���ˮ����Һ��Ϊ��ɫ | ����Һ��һ������Fe2+ |
| C | ��ij����ͨ��Ʒ����Һ�У�Ʒ����Һ��ɫ | ������һ����SO2 |
| D | ȡ1mL20%��������Һ������3��5��ϡ���ᣬˮԡ����5min��ֱ�Ӽ����������Ʊ���Cu��OH��2������3��5min��û�в���ש��ɫ���� | ˵������û��ˮ�⣮ |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |
| A�� | ʵ�����ͨ��Ũ���������ɷ�ΪCO2��H2 | |
| B�� | ���������һ������H2S��H2��CO2��������O2 | |
| C�� | ���л�ѧ����ʽ�ɱ�ʾH2S+Cu�TCuS+H2 | |
| D�� | �ѻ���������������壬���ܷ���ɿ��֣��������������� |