��Ŀ����
��12�֣���ȸʯ��Ҫ��Cu2(OH)2CO3����������Fe��Si�Ļ����ʵ�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O��CaCO3���������£�

��ش��������⣺
(1)��ҺA�еĽ���������Cu2����Fe2����Fe3���������������Լ���ѡ��ʵ�鲽�����Լ���Ϊ________(�����)��������ҺA��Fe3��������Լ�Ϊ________(�����)��
a��KMnO4 b��(NH4)2S c��H2O2 d��KSCN
(2)����ҺC���CuSO4��5H2O����Ҫ��������������________�����˵Ȳ��������ձ���©���⣬���˲������õ���һ�����������������ڴ˲����е���Ҫ������___________��
(3)�Ʊ�CaCO3ʱ��Ӧ��CaCl2��Һ����ͨ��(���ȼ���)________(�ѧʽ)����ʵ��������а����ݳ���Ӧѡ������________(�����)װ�û��ա�

��ش��������⣺
(1)��ҺA�еĽ���������Cu2����Fe2����Fe3���������������Լ���ѡ��ʵ�鲽�����Լ���Ϊ________(�����)��������ҺA��Fe3��������Լ�Ϊ________(�����)��
a��KMnO4 b��(NH4)2S c��H2O2 d��KSCN
(2)����ҺC���CuSO4��5H2O����Ҫ��������������________�����˵Ȳ��������ձ���©���⣬���˲������õ���һ�����������������ڴ˲����е���Ҫ������___________��
(3)�Ʊ�CaCO3ʱ��Ӧ��CaCl2��Һ����ͨ��(���ȼ���)________(�ѧʽ)����ʵ��������а����ݳ���Ӧѡ������________(�����)װ�û��ա�

��1��c d ��2����ȴ�ᾧ ���� ��3��NH3 b
��1��Ҫ��ȡ����ͭ���壬�ͱ����ȥ��Һ�е��������ӣ����ó�������ȥ�����ӡ��������Ҫ����Һ�е����������������������ӣ���ͬʱ�ֲ��������µ����ʣ�˫��ˮ�Ļ�ԭ������ˮ�������������ʡ����Դ�ѡC��������ҺA��Fe3��������Լ���KSCN��Һ��
��2��Ҫ���CuSO4��5H2O����Ҫͨ��������������ȴ�ᾧ��Ȼ����˼��ɡ�����ʱ���ձ���©���⣬����Ҫ������������
��3������CO2���ܽ�Ⱥ�С����������ͨ�백����ʱ��Һ�Լ��ԣ�Ȼ����ͨ��CO2���õ�̼��ƹ��塣���ڰ�����������ˮ������������Ҫ������ֹ��������˴�ѡB��
��2��Ҫ���CuSO4��5H2O����Ҫͨ��������������ȴ�ᾧ��Ȼ����˼��ɡ�����ʱ���ձ���©���⣬����Ҫ������������
��3������CO2���ܽ�Ⱥ�С����������ͨ�백����ʱ��Һ�Լ��ԣ�Ȼ����ͨ��CO2���õ�̼��ƹ��塣���ڰ�����������ˮ������������Ҫ������ֹ��������˴�ѡB��

��ϰ��ϵ�д�
 �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
�����Ŀ
 H2SO4��Ũ��
H2SO4��Ũ��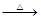 Li2SO4
Li2SO4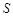 (Li2CO3)/g
(Li2CO3)/g

