��Ŀ����
8�������йػ�ѧ����ʹ����ȷ���ǣ�������| A�� | ̼�����Ƶ�ˮ�ⷽ��ʽ��HCO3-+H2O=CO32-+H3O+ | |
| B�� | ��ϡ��Һ�У�����Ӧ����1molˮʱ�ų�57.3KJ����������ϡ���������ʯ��ˮ�����кͷ�Ӧ���Ȼ�ѧ����ʽΪH+��aq��+OH-��aq���TH2O��l����H=-57.3kJ•mol-1 | |
| C�� | ��NaHSO4��Һ�м������Ba��OH��2��Һ�����ӷ���ʽ��2H++SO42-+Ba2++2OH-�TBaSO4��+2H2O | |
| D�� | ��Na2CO3��Һ��������SO2�����ӷ���ʽ��CO32-+SO2�TCO2+SO32- |
���� A��̼��������Ӳ���ˮ������̼������������ӣ���Һ��ʾ���ԣ�
B���������ʵ����ʵ���������֮��Ĺ�ϵ�Լ��Ȼ�ѧ����ʽ����д������д��
C����NaHSO4��Һ�м������Ba��OH��2��Һ��NaHSO4��Һ��Ӧ��ȫ��
D�����������ˮ��Ӧ���������ᣬSO2������̼������Ӵ�������Ӧ����̼��������ӣ�
��� �⣺A��̼�����Ƶ������̼��������Ӳ���ˮ������̼������������ӣ���ˮ������ӷ���ʽΪ��HCO3-+H2O?H2CO3+OH-����A����
B��ϡǿ�ᡢϡǿ�Ӧ����1molˮʱ�ų�57.3kJ��������ϡ���������ʯ��ˮ����ǿ���ǿ���ϡ��Һ����Ӧ���Ȼ�ѧ����ʽΪ��H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ•mol-1����B��ȷ��
C��NaHSO4��Һ����������NaHSO4��Һ��Ӧ��ȫ���������ӷ���ʽΪ��Ba2++OH-+H++SO42-�TBaSO4��+H2O����C����
D��SO2������̼������Ӵ�������̼������Һ��������������������ӷ�ӦΪ��2CO32-+SO2+H2O�T2HCO3-+SO32-����D����
��ѡB��
���� ���⿼�����ӷ���ʽ����д����Ŀ�Ѷ��еȣ�ע��ӻ�ѧʽ�����ӷ��š�����غ��Լ��Ƿ���Ϸ�Ӧʵ�ʵĽǶȷ�����D��Ӧ����̼���������Ϊ�״��㣮

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�| A�� | ��λ���ʵ�����������ռ�������������Ħ����� | |
| B�� | ͨ��״�������¡�101kP���£�����Ħ�����ԼΪ22.4 L/mol | |
| C�� | ��״����0�桢101kP���£�����Ħ�����ԼΪ22.4 L/mol | |
| D�� | ��״����0�桢101kP���£�1 mol H2O�������22.4 L |
| A�� | 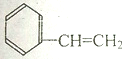 | B�� | 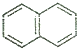 | C�� |  | D�� | 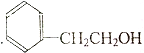 |
| A�� | $\frac{m}{V}$mol•L-1 | B�� | $\frac{m}{24V}$mol•L-1 | C�� | $\frac{m}{12V}$mol•L-1 | D�� | $\frac{m}{48V}$mol•L-1 |
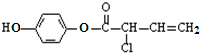 �й�M��������������ȷ���ǣ�������
�й�M��������������ȷ���ǣ�������| A�� | ����ʽΪC10H11O3Cl | |
| B�� | һ�������¿��Է���ȡ����Ӧ���ӳɷ�Ӧ��������Ӧ | |
| C�� | ����NaOH��Һ��Ӧ��1mol M�������4mol NaOH | |
| D�� | ������FeCl3������ɫ��Ӧ��Ҳ�ܺ� Na2CO3��Һ��Ӧ�ų�CO2�� |
| A�� | NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l������H=+28.7 kJ•mol-1 | |
| B�� | NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l������H=-28.7 kJ•mol-1 | |
| C�� | NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l������H=+57.4 kJ•mol-1 | |
| D�� | NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l������H=-57.4 kJ•mol-1 |
| ���� �� | IA | 0 | ||||||
| 1 | �� | IIA | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� | ||||||
��2����ЩԪ�ص�����������Ӧˮ������������ǿ����HClO4��������ǿ����KOH���ѧʽ��
��3����̬�⻯������������������Ӧ��ˮ���ﷴӦ�����εĻ�ѧ����ʽΪ��NH3+HNO3=NH4NO3��
��4�������Ե���������ֱ���ܺ͢�����������Ӧ��ˮ���ﷴӦ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��Al��OH��3+3H+=Al3++3H2O��
| A�� | ���� | B�� | ��ϩ | C�� | �� | D�� | �Ҵ� |
��1��NԪ��λ�����ڱ��ڶ����ڣ��ڢ�A�壬CԪ�ص�һ��ͬλ�ؿɲⶨ�������������ͬλ�صķ�����146C��
��2���á���������������=����գ�
| ���Ӱ뾶 | �õ������� | ���� | ������ |
| O2-��Al3+ | 16O=18O | H2CO3��HNO3 | Fe��Al |
��4��ClO2������ˮ�ľ�������ҵ�Ͽ���Cl2����NaClO2��Һ��ȡClO2��д���÷�Ӧ�����ӷ���ʽ�����õ����ű������ת�Ƶķ������Ŀ
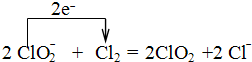 ��
����5������Ƭ�������ȥ����Ĥ����̼���õ������Ӻ����ϡNaOH��Һ�п��Թ���ԭ��أ��������Ϊ̼������ظ�����Ӧ�ĵ缫����ʽΪAl-3e-+4OH-=AlO2-+2H2O��