��Ŀ����
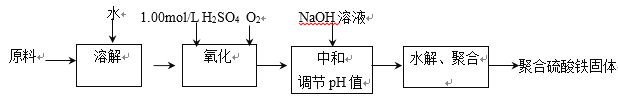
����Ŀ����ˮ��һ�ַḻ����Դ����ҵ�������ԴӺ�ˮ����ȡ�������õ����ʣ���Щ���ʹ㷺Ӧ��������������Ƽ��ȷ��档��ͼ�ǴӺ���CCl4��Һ�õ����ʵ����̣�
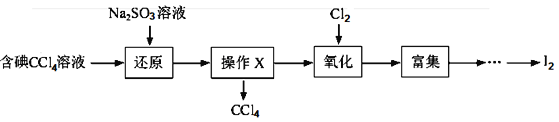
���������գ�
��1������Һ�м����Թ�����Na2SO3��Һ�������ӷ���ʽΪ_________���ò�����I2��ԭΪI����Ŀ����__________��
��2������X������Ϊ_______�����õ���Ҫ������_________��
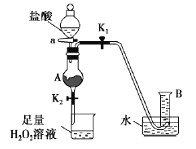
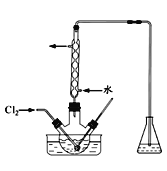
��3������ʱ��������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����400C���ҷ�Ӧ��ʵ��װ����ͼ��ʾ����ʵ������ڽϵ��¶��½��е�ԭ����_________����ƿ��ʢ�ŵ���ҺΪ________��
��4����֪��5SO32��+2IO3��+2H�� �� I2+5SO42��+H2Oij������Һ��pHԼΪ8����һ������I2�����ܴ���I����IO3���е�һ�ֻ����֡��벹���������麬����Һ���Ƿ�ͬʱ����I����IO3����ʵ�鷽����_______��ʵ���пɹ�ѡ����Լ���CCl4��ϡ���ᡢ������Һ
��5��Ũ��ˮ��ȡþ�Ĺ�����������ͼ��
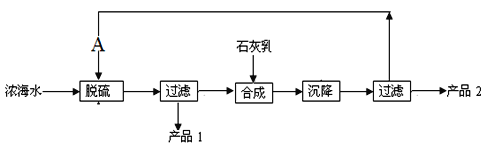
Ũ��ˮ����Ҫ�ɷ����£�
���� | Na+ | Mg2+ | Cl- | SO42- |
Ũ��/��g��L-1�� | 63.7 | 28.8 | 144.6 | 46.4 |
�ù��չ����У��������Ҫ��Ӧ�����ӷ���ʽΪ________����Ʒ2�Ļ�ѧʽΪ__________��1LŨ��ˮ���ɵõ���Ʒ2������Ϊ____g��
���𰸡�SO32-+I2+H2O �� 2I-+SO42-+2H+ ʹ���Ȼ�̼���еĵⵥ��ת��Ϊ�����ӽ�����Һ ��Һ ��Һ©�� ��ֹ���������߱������������������ܽ�� ����������Һ ȡ����������Һ��CCl4�����ȡ����Һ��ˮ���õ�����Һ���鲻���ⵥ�ʴ��ڣ���ˮ��ȡ������Һ������1-2ml������Һ���������ữ������Һ������˵����ˮ��ͬʱ���е����Ӻ͵����������Һ��������˵����ͬʱ�������Ӻ͵���� Ca2+ + SO42- = CaSO4�� Mg��OH��2 69.6��
��������
�����ǿ�������ܽ����������������Ϊ��������ӣ���������ԭ���ɵ����ӣ����ԭΪ�����ӣ�Ŀ�������÷�Һ����ȥ���Ȼ�̼�����뻥�����ܵ�Һ����÷�Һ������ʹ�õ������Ƿ�Һ©�����ⲻ�ȶ��������������¶ȸ�ʱ�������ܽ⣻���ܺ�ǿ����Һ��Ӧ���������Ȼ�̼��Һ��ȡ������Һ��Ȼ���Һ��ˮ���õ�����Һ���鲻����ʱ,ȡˮ����Һ������Һ�м��������Һ���ټ������ữ��������Һ�Ƿ����ɫ�жϣ��������̺ϳɲ����м���ʯ���飬���������˺����Һ��������Ӧ�ø����ӳ��������������������Ƴ�������Ʒ1Ϊ����ƣ��ϳɵõ�������þ�����ʹ��˺����IJ�Ʒ2Ϊ������þ������1L��Һ��Mg2+������������Mg2+---Mg(OH)2����������þ���������ݴ˷�����
(1)�����ǿ�������ܽ����������������Ϊ��������ӣ���������ԭ���ɵ����ӣ����ӷ���ʽΪSO32-+I2+H2O=2I-+SO42-+2H+�����ԭΪ�����ӣ�Ŀ�������÷�Һ����ȥ���Ȼ�̼�����Ըò�����I2��ԭΪI-��Ŀ����ʹ���Ȼ�̼�еĵⵥ��ת��Ϊ�����ӽ�����Һ�����ǣ�SO32-+I2+H2O=2I-+SO42-+2H+��ʹ���Ȼ�̼���еĵⵥ��ת��Ϊ�����ӽ�����Һ��
(2)���뻥�����ܵ�Һ����÷�Һ�������������Ȼ�̼��ˮ��Һ���÷�Һ����������X����Ϊ��Һ��ʹ�õ������Ƿ�Һ©�������ǣ���Һ����Һ©����
(3)�ⲻ�ȶ������������¶ȸ�ʱ�������ܽ⣬�����¶Ƚϵ͵�ԭ���ǣ���ֹ���������߱����������������ܽ�ȣ����ܺ�ǿ����Һ��Ӧ����ƿ��Һ����NaOH��Һ�����ǣ���ֹ���������߱����������������ܽ�ȣ�NaOH��Һ��
(4)�������Ȼ�̼��Һ��ȡ������ҺȻ���Һ��ˮ���õ�����Һ���鲻����ʱ,Ȼ��ȡˮ����Һ������Һ�м��������Һ���ټ������ữ����е����Ӻ͵�������ӣ����߷���������ԭ��Ӧ���ɵⵥ�ʣ�������Һ����ɫ������ͬʱ���е����Ӻ͵�������ӣ����ǣ�ȡ����������Һ��CCl4�����ȡ����Һ��ˮ���õ�����Һ���鲻���ⵥ�ʴ��ڣ���ˮ��ȡ������Һ������1-2mL������Һ���������ữ������Һ������˵����ˮ��ͬʱ���е����Ӻ͵����������Һ��������˵����ͬʱ�������Ӻ͵������
(5)�������̺ϳɲ����м���ʯ���飬���������˺����Һ����������Ca2+����SO42-����CaSO4���������ӷ���ʽΪ��Ca2++SO42-=CaSO4�������ù��˵ķ����õ���Ʒ1ΪCaSO4����Һ�м���ʯ���飬������ӦΪMg2++2OH-=Mg(OH)2�����ϳ���Ӧ�õ�Mg(OH)2���������ˡ�����IJ�Ʒ2ΪMg(OH)2����Һ��m(Mg2+)=1L��28.8g�ML=28.8g����þ���Ӻ�������þ��ϵʽ��Mg2+Mg(OH)2����m(Mg(OH)2)=28.8g��58��24=69.6g�����ǣ�Ca2++SO42-=CaSO4����Mg(OH)2��69.6�ˡ�

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�����Ŀ��ij��ʵ������ȡ���ᶡ������ԭ��Ϊ��7.4 mL1-������6.0 mL�����ᣬ1.0mLŨ���ᡣ
1-���� | ������ | |
�ܶȣ�g/cm3�� | 0.81 | 1.05 |
Ħ������(g/mol) | 74 | 60 |
���Ƶ����ᶡ����ʽ��116��������Ϊ5.12 g����������ȷ����
A.���ʣ�Լ54.49%B.���ʣ�Լ42.04%
C.ת���ʣ�������С��1-����D.ת���ʣ����������1-����
����Ŀ����1�������£���0.5mol/L�Ĵ�����Һ�м�������ˮ����ˮ�������c(H+)��(OH-)___(���������������С������������)��
��2����֪Ksp(Ag2CrO4) =1.0��10-12����0.2mol/L��AgNO3��Һ�м���������0.008mol/LK2CrO4��Һ�� ����Һ�е�c(CrO42-)=___��
��3�������£�0.1mol/LNaHCO3��Һ��pHֵ___0.1mol/LNa2SO3��Һ��pHֵ(����>������<������=")��
H2CO3 | K1=4.3��10-7 | K2=5.6��10-11 |
H2SO3 | K1=1.54��10-2 | K2=1.02��10-7 |
��֪��
��4����һ�ֿɳ����Na��Al/FeS����ع���ʱNa+�����ʵ������ֲ��䣬�������ú�Na+�ĵ��������Ϊ����ʣ���֪�õ��������ӦʽΪ2Na++FeS+2e-=Na2S+Fe����õ���ڳ��ʱ������������Ӧ��������___���ŵ�ʱ������ӦʽΪ___��