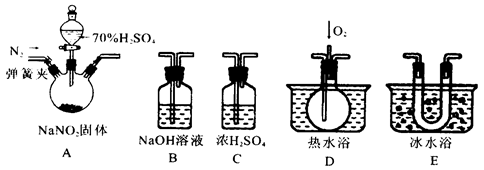
��Ŀ����
�������Ũ�������ܷ����ۻ�����ij��ȤС���ͬѧ���ֽ�һ����������Ũ�������ʱ���۲쵽����ȫ�ܽ⣬�������������塣Ϊ�ˣ��������������װ����֤�����������塣
��1��֤����Ӧ����������������SO2���ɵ������� ��
��2��֤�������к���������ʵ�������� ��
��3��Ϊ�˽�һ��̽����Ӧ��A��Һ����Ԫ�صļ�̬�����ǽ��������µļ��裺
����1����Һ����Ԫ�ؼ���Fe3+Ҳ��Fe2+
����2����Һ����Ԫ��ֻ��Fe3+
����3����Һ����Ԫ��ֻ��________________
���ڼ���1�������Լ���0.01 mol/L����KMnO4��Һ��ϡ��ˮ��Һ��0.1 mal/L KI��Һ��
������Һ��KSCN��Һ������ˮ����̽����������Һ������ɱ������ݡ�
��ʵ��̽����
| ʵ����� | Ԥ������ | ���� |
| ȡ��Ӧ���A��Һ��װ��a��b���Թܣ�����٣���a�Թ��е��� �� | | |
| ����ڣ���b�Թ��е��� �� | | ��Һ����Fe3+ |
��16�֣���1��Ʒ����Һ��dz������ɫ�� ��2�֣�
��2��E�к�ɫ(CuO)��ɺ�ɫ��F�а�ɫ��ĩ(CuSO4)�����ɫ����2�֣�
��3������3����Һ����Ԫ��ֻ��Fe2+ ��2�֣�
��ʵ��̽����ʵ����� Ԥ������ ���� ȡ��Ӧ���A��Һ��װ��a��b���Թܣ�����٣���a�Թ��е���������1�֣�0.01mol/L����KMnO4��Һ��1�֣��� KMnO4��Һ���Ϻ�ɫ��ȥ�����dz����2�֣� ��Һ����Fe2+��2�֣� ����ڣ���b�Թ��е������Σ�1�֣�KSCN��Һ�������1�֣�[�Σ���������0.1mol/LKI��Һ�͵�����Һ��1�֣�] �� ��Һ��ΪѪ��ɫ��2�֣�
[����Һ��Ϊ��ɫ��2�֣�]��Һ����Fe3+
���������������1��SO2����Ư���ԡ����ԡ���ԭ�Ժ������ԣ���ͼ��֪����ʵ����Ʒ����Һ�����Ƿ���SO2���ɣ���Ʒ����Һ��ɫ���dz��˵��װ��A�з�Ӧ���������庬��SO2����2����������ǿ��ԭ�ԣ��ڼ�����������ʹ��ɫ������ͭ��ԭΪ��ɫ�ĵ���ͭ��H2������Ϊ��ʹ��ɫ����ˮCuSO4���������H2O����װ��E�к�ɫ�����Ϊ��ɫ��F�а�ɫ��ĩ��Ϊ��ɫ��˵��װ��A�зų��������к���H2����3������������Ũ����ۻ���������һ�����������ﱡĤ��������ǿ�����ԡ�ǿ���Ե�Ũ������Խ�������Ϊ���Σ��������ĵ��������Խ����λ�ԭΪ�����Σ���˷�Ӧ��A����Һ����Ԫ�ؿ��ܼ���Fe2+����Fe3+��Ҳ����ֻ��Fe3+��������ֻ��Fe2+��������֪����1������2����Ϣ�ƶϼ���3Ϊ��Һ����Ԫ��ֻ��Fe2+�����������仯��������ʿ�֪��Fe2+���л�ԭ�ԣ���ʹ����KMnO4��Һ��ɫ��Fe3+���������ԣ��ܽ�KI��Һ��ԭΪ��ʹ������Һ������I2��Fe2+��KSCN��Һ����죬��Fe3+��KSCN��Һ��죬��������Fe3+����Һ�ʻ�ɫ������ˮ����ɫ��ͬ���ɴ˿���ѡ���ʵ����Լ����ʵ�鷽����֤A����Һ�м���Fe2+����Fe3+��
���㣺����ʵ�鷽������ƣ��漰Ԫ�ػ���������ʡ�����ļ��鷽����������衢���ʵ�鷽����֤�����֪ʶ��

Ŀǰ���еĹ���������Դ�����������Ϊ,������Դ��Լ40����ǰ�Ĺ������Һ���������ֻ���ϵͳ���ձ��������ؽṹ,��Fe2S2��Fe4S4��Fe8S7��,��Щ����ؽṹ������������Դ����ط�Ӧ��ij��ѧ��ȤС�����о�ij����ؽṹ�����ʱ,���������ʵ�顣
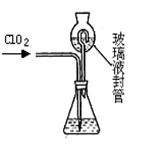
��ʵ��� �������ȷ��: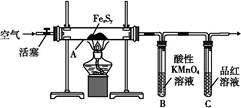
��ͼ����װ��,����װ�õ������Ժ�,��Ӳ�ʲ�����A�з���1.0 g����ؽṹ(���в��ֲ���Ӧ������),���Թ�B�м���50 mL 0.1 mol��L-1������KMnO4��Һ,���Թ�C�м���Ʒ����Һ��ͨ�����������,���ֹ�����ת��Ϊ����ɫ����������ȫת����B����Һת����250 mL����ƿ,ϴ���Թ�B���ݡ�ȡ25.00 mL����Һ��0.01 mol��L-1�IJ���(H2C2O4)���вⶨʣ��KMnO4��ҺŨ�ȵĵζ�����¼��������:
| �ζ����� | ������Һ ���/mL | ������Һ���/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 1.50 | 23.70 |
| 2 | 25.00 | 1.02 | 26.03 |
| 3 | 25.00 | 0.00 | 24.99 |
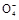 +2H2O+5SO2
+2H2O+5SO2 2Mn2++5S
2Mn2++5S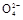 +4H+
+4H+��2Mn
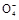 +6H++5H2C2O4
+6H++5H2C2O4 2Mn2++10CO2��+8H2O
2Mn2++10CO2��+8H2O��ʵ��� ��������ȷ��:
��ʵ���Ӳ�ʲ�����A�еIJ����������ϡ������,��ֽ�������,����Һ�м���������NaOH��Һ,���˺�ȡ����,��������յ�0.6 g���塣
�Իش���������:
(1)��顰ʵ�����װ�������Եķ����� ��
(2)�ζ��յ���жϷ����� ��
(3)�Թ�C��Ʒ����Һ�������� ��
��ͬѧ���,��ȥCװ��,��ʵ��û��Ӱ��,��Ŀ������������������������� (ѡ�ͬ�⡱��ͬ�⡱),������ ��
(4)����ʵ����ʵ����е����ݿ�ȷ��������ؽṹ�Ļ�ѧʽΪ ��
������̽���� �ζ�������,ϸ�ĵ�С�����ָ�KMnO4��ɫ��ȥ�����ʽ�ƽ���ζ�ʱҪ��öࡣΪ�о����ԭ��,��ͬѧ��������������ʵ��,ʵ���������±�:
| ��� | �¶�/�� | �ữ��H2C2O4 ��Һ/mL | KMnO4 ��Һ/mL | ��Һ�� ɫʱ��/s |
| 1 | 25 | 5.0 | 2.0 | 40 |
| 2 | 25 | 5.0(������������ ��ˮ��MnSO4��ĩ) | 2.0 | 4 |
| 3 | 60 | 5.0 | 2.0 | 25 |
(5)������������,�ζ������з�Ӧ���ʽϿ��һ�ֿ���ԭ���� ��
��1��ij��������������ŷŵ���ʯ���������������ˮ�࣬���᷵������������李�����������ͼ���£�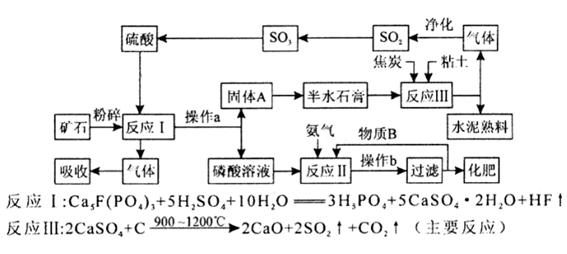
�ٲ���b��������Ũ������ȴ�� ��
���練ӦII�IJ�����������ʽ�Σ�������B�����ʵĻ�ѧʽ�� ��
������ʵ�����жԷ�ӦII������������ ���ա�
�ܸ��������������ŵ��Ǿ����ܵ�ʵ��ԭ�ϵ�ѭ��ʹ�ú�������ۺ����ã��������������ֵĻ���˼���� ��
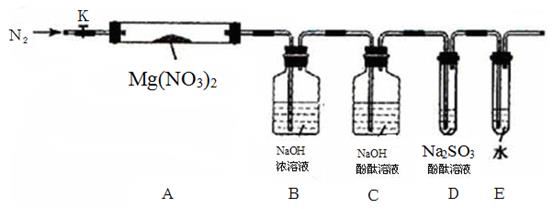
��2��Ϊ�ⶨͬ����炙������̬��������������ʵ��������ͼ��ʾװ�ý���ʵ�顣ʵ��ʱ����A�м���mg�����Ʒ���ر�ֹˮ��a����ֹˮ��b����A�м���������ŨNaOH��Һ����ȫ��Ӧ��C��Ũ��������ng����ش��������⣺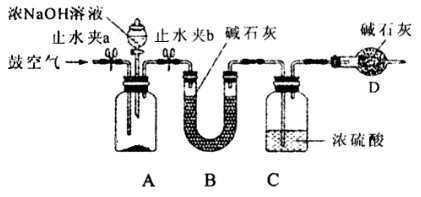
����˵������װ�������ԵIJ���������ʵ������ ��
��װ��B�������� ��װ��D�������� ��
��ʵ�����Ӧ���ں�ʱ�������?�� (���ʼǰ�����������С���Ӧ��)
����ij�βⶨ���̬����������������ƫ�ͣ�����ܵ�ԭ���� (����ĸ)��
| A�������Ʒ����������δ��ַ�Ӧ | B��A��B�в�����һ�����İ��� |
| C������������Һ��Ũ��̫�� | D���������� |
ij��ȤС�����þ���ˮ�ķ�Ӧ�Ʋ�þҲ���뱥��̼��������Һ��Ӧ��������ʾ��þ�뱥��̼��������Һ��Ӧ������������Ͱ�ɫ���������ȤС�����������ʵ�鷽������֤���̽����Ӧԭ����
ʵ��1����ɰֽ��ȥþ����������Ĥ���������ʢ���������з�̪�ı���̼��������Һ���Թ��У�Ѹ�ٷ�Ӧ�������������ݺͰ�ɫ�������Һ��dz���졣
��1���������
��ͬѧ�Է�Ӧ�в����İ�ɫ�������������¼��裺
����1������Ϊ ��
����2������ΪMgCO3��
����3�������Ǽ�ʽ̼��þ[xMgCO3��yMg(OH)2]
��2����ƶ���ʵ��ȷ�����ﲢ��֤�²⣺
| ʵ����� | ʵ�� | Ԥ������ͽ��� |
| ʵ��II | ��ʵ��I���ռ����������ȼ | |
| ʵ��III | ȡʵ��I�еİ�ɫ�����ϴ�ӣ��������� | �� ��ɫ��������ܺ���MgCO3 |
| ʵ��IV | ȡʵ��I�еij���Һ�������м�������CaCl2ϡ��Һ | ������ɫ��������Һ�д��� ���� |
��3����ƶ���ʵ��ȷ��ʵ��I�IJ����ȡʵ��I�����ø�������İ�ɫ������31.0 g����ּ��������ٲ�������Ϊֹ����ʹ�ֽ����������ȫ������ʢ������Ũ�����ϴ��ƿA��ʢ��������ʯ�ҵĸ����B��ʢ��������ʯ�ҵĸ����C�С�ʵ��ǰ��װ��A����1.8 g��װ��B����13.2 g����ȷ����ɫ������Ļ�ѧʽ ��
��4�����ϻ�ѧ����ͻ�ѧƽ���ƶ�ԭ������Mg��NaHCO3��Һ��Ӧ�����������ݵ�ԭ�� ��
��ѧ������������ء�����˵������ȷ����
| A����ϩ����ˮ���Ĵ���� |
| B���轺������װʳƷ�ĸ���� |
| C���������ֿ���ʳƷ�ı��ʼ� |
| D��������������θ����кͼ� |
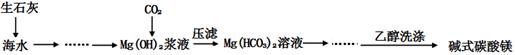
 ��x+y��MgO+xCO2��+��y+z��H2O
��x+y��MgO+xCO2��+��y+z��H2O