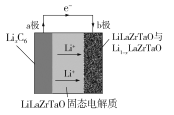
��Ŀ����
����Ŀ���ڷ�����ѧ�ĵ�λ���У��ʹ��缫�����αȵ缫�������ɽ���������������Hg2Cl2��KCl��Һ��ɵĵ缫��Hg2Cl2���ʹ������Խ�С����HgCl2���������о綾��
��1��KԪ�صĻ�̬ԭ�ӵĵ��������_____����ͬ���ܼ���
��2��Hg�ļ۲�����Ų�ʽΪ5d106s2��HgԪ��λ��Ԫ�����ڱ���_______����
��3��Hg2Cl2��400~500��ʱ�������ɴ��Ʋ�Hg2Cl2�ľ�������Ϊ____��
��4��KCl��NaCl��ȣ�____ ���۵���ߣ�ԭ����________��
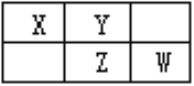
��5����NH4Cl��HgCl2��һ��������ϣ����ܷ���м���ʱ������ij�־��壬�侧����ͼ��ʾ����X-�������䷨��øþ���ľ���Ϊ�����壨��������a=b =419pm��c=794pm����ÿ��NH4+����Ϊ��8��Cl-Χ�ƣ�����Ϊ335pm��Cl-��Cl-������Զ�롣

�ٸþ���Ļ�ѧʽΪ________��
�ھ�����Cl-�Ŀռ价��_____________�����ͬ������ͬ����������������˵������_______________
���谢���ӵ�������ֵΪNA����þ�����ܶ�Ϊ_______g/cm3���г��������ʽ����
���𰸡�6 ds ���Ӿ��� NaCl ���߾�Ϊ���Ӿ��壬�������������ͬ��Na+�뾶��K+С��NaCl�ľ����ܸ����۵���� NH4HgCl3 ����ͬ ���ĵ�Cl-����NH4+Ϊ335pm�����ĵ�Cl-����NH4+Ϊ397pm 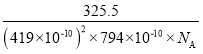
��������
(1)KԪ�صĻ�̬ԭ�Ӻ�����19�����ӣ���������Ų�ʽΪ1s22s22p63s23p64s1���������6����ͬ���ܼ����ʴ�Ϊ��6��
(2)Hg�ļ۲�����Ų�ʽΪ5d106s2����HgΪ�������ڵ���B��Ԫ�أ�λ��Ԫ�����ڱ���ds�����ʴ�Ϊ��ds��
(3)Hg2Cl2��400~500��ʱ�������۷е�ϵͣ�����Ϊ���Ӿ��壬�ʴ�Ϊ�����Ӿ��壻
(4)����NaCl��KCl��Ϊ���Ӿ��壬�������������ͬ��Na+�뾶��K+С����NaCl�ľ����ܸ����۵���ߣ��ʴ�Ϊ��NaCl�����߾�Ϊ���Ӿ��壬�������������ͬ��Na+�뾶��K+С��NaCl�ľ����ܸ����۵���ߣ�
(5)���ɾ���ʾ��ͼ��֪��NH4+λ�ھ����Ķ��㣬һ�������к���![]() ��NH4+��Cl-λ�ھ��������Ϻ����ģ�һ�������к���
��NH4+��Cl-λ�ھ��������Ϻ����ģ�һ�������к���![]() ��Cl-��Hg2+λ�ھ��������ģ�һ�������к���1��Hg2+����þ���Ļ�ѧʽΪNH4HgCl3���ʴ�Ϊ��NH4HgCl3��
��Cl-��Hg2+λ�ھ��������ģ�һ�������к���1��Hg2+����þ���Ļ�ѧʽΪNH4HgCl3���ʴ�Ϊ��NH4HgCl3��
���������Ϣ��֪�����ĵ�Cl-����NH4+Ϊ335pm�����ĵ�Cl-����NH4+Ϊ![]() ��������Cl-�Ŀռ价������ͬ���ʴ�Ϊ������ͬ�����ĵ�Cl-����NH4+Ϊ335pm�����ĵ�Cl-����NH4+Ϊ397pm��
��������Cl-�Ŀռ价������ͬ���ʴ�Ϊ������ͬ�����ĵ�Cl-����NH4+Ϊ335pm�����ĵ�Cl-����NH4+Ϊ397pm��
�۸��ݹ�ʽ![]() �ɵã��þ�����ܶ�
�ɵã��þ�����ܶ�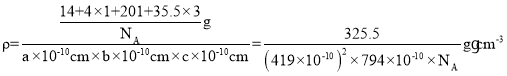 ���ʴ�Ϊ��
���ʴ�Ϊ��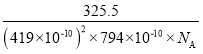 ��
��

 ��������һ���þ�ϵ�д�
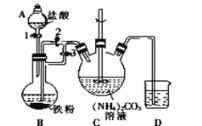
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
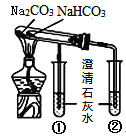
Сѧ��10����Ӧ����ϵ�д�����Ŀ���������ʵ����ѡ���װ�û�����(�г�װ������ȥ)��ȷ���ǣ� ��
A | B | C | D | |
ʵ�� | ����ƾ���ˮ | ���뽺�����Һ | ��ȥ̼���ƹ����е�̼������ | �Ƚ�̼���ƺ�̼�����Ƶ��ȶ��� |
װ�û����� |
|
|
|
|
A.AB.BC.CD.D