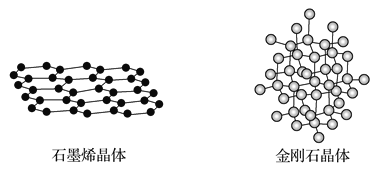
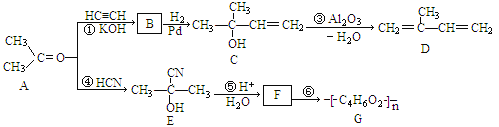
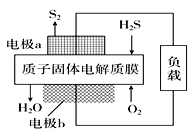
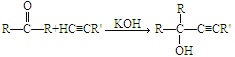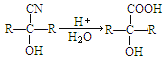��Ŀ����
����Ŀ��ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)���������£�
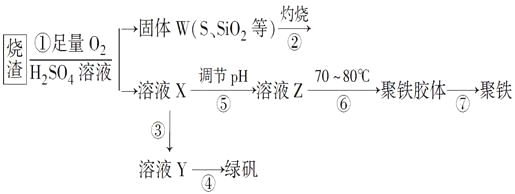
��1�����������еIJ���������ͨ��������Һ�У���Һ����ɫ����________��
A��Ʒ����Һ B����ɫʯ����Һ C������KMnO4��Һ D����ˮ
��2���������У�FeS��O2��H2SO4��Ӧ�����ӷ���ʽΪ��________��
��3����������������������________��
��4���������У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ��������___________��
��5������������pH��ѡ�������Լ��е�________(��ѡ�����)��
A��ϡ���� B��CaCO3 C��NaOH��Һ
��6���������У�����ҺZ���ȵ�70��80����Ŀ����___________��
��7��ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ2.800 g��Ʒ��������Ʒ�����������������������Ȼ�����Һ�������ˡ�ϴ�ӡ�����������ù�������Ϊ3.495 g�����þ�����Ҫ�ɷ�Ϊ[Fe(OH)(SO4)]n����þ�����Ʒ����Ԫ�ص���������Ϊ________��(���������в�����Ԫ�غ���Ԫ��)��
���𰸡�ACD 4FeS��3O2��12H��=4Fe3����6H2O��4S Fe(����) ���������� C �ٽ�Fe3����ˮ�� 30%
��������
�����̿�֪��������������Һ��ͨ��������������Ӧ����˵õ�����W����ҺX��������ҺX��������Ӧ����������������������Ũ���ᾧ�����õ��̷���������ҺX����NaOH������ҺpH�õ���ҺZ����70��-80���¼����Ʊ��������壬���õ���������ʽ�������ľۺ����
��1�����̢ڲ���������ΪSO2�����л�ԭ�Ժ�Ư���ԣ������Ư���Զ�ʹƷ����ɫ������л�ԭ�Զ�ʹ���������Ե�����KMnO4��Һ����ˮ��ɫ���ʴ�Ϊ��ACD��
��2����Ӧ��ΪFeS��O2��H2SO4����������S�����������غ㻹Ӧ��Fe2��SO4��3��H2O���ɵ��Ӽ�ԭ���غ��֪��Ӧ�����ӷ���ʽΪ4FeS��3O2��12H��=4Fe3����6H2O��4S���ʴ�Ϊ��4FeS��3O2��12H��=4Fe3����6H2O��4S��
��3����ҺX�к���Fe3+��Ҫ�Ʊ��̷���Ӧ���뻹ԭ��ʹ֮��ԭΪFe2+��������������Fe2+�����Ҳ������µ����ʣ��ʴ�Ϊ��Fe(����)��
��4�����̢��У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ���������������������ʴ�Ϊ��������������
��5�����̢ݵ���pHӦʹ��Һ������ǿ�������ھ��������ɣ�
A.���������ʹ��ҺpH����A����
B. ����̼��������ܵ�����ƣ��������µ����ʣ���B����
C.����NaOH��ʹ��Һ������ǿ�����ɵľ����в������µ����ʣ���C��ȷ��
�ʴ�Ϊ��C��
��6�����̢��У�����ҺZ���ȵ�70-80�棬Ŀ���Ǵٽ�Fe3+��ˮ�⣬�ʴ�Ϊ���ٽ�Fe3+��ˮ�⣻
��7���ⶨ��Ԫ�ص���������ʱ�������Ȼ�����Һ�������Ĺ���Ϊ���ᱵ���������������SO42-�����ʵ���Ϊ0.015mol�����ݾ����Ļ�ѧʽ��ȷ��Fe�����ʵ���Ϊ0.015mol���������������Ϊ0.84g������������Ϊ0.84g��2.800 g ��100%=30%���ʴ�Ϊ��30%��

����Ŀ����ͼװ�ÿ������ռ�SO2����֤��ijЩ��ѧ���ʣ�����˵����ȷ����
ѡ�� | �Լ� | ���� | ���� |
A | ���Ը��������Һ | ��Һ��ɫ | SO2��Ư���� |
B | Ʒ����Һ | ��Һ��ɫ | SO2�������� |
C | ���з�̪��NaOH��Һ | ��Һ��ɫ | SO2�л�ԭ�� |
D | H2Sˮ��Һ | ��Һ����� | SO2�������� |
A.AB.BC.CD.D
����Ŀ����������ʵ���������ý�������ȷ����
ʵ�� | ʵ������ |
| ��Ʒ����Һ��ɫ��ȥ����������Һ�к���SO42- �ڼ�����ɫ�����Һ�����ڣ�δ����ɫ�ָ����������������ʹ��ɫʯ����ֽ��죬�����۵⻯����ֽ�������Ա仯�� |
A. Ʒ����Һ��ɫ����ˮƯ������
B. ����ƿ�з����˷�Ӧ��Cl2 + SO2 + 2H2O H2SO4 + 2HCl
C. ��������ڿ�ȷ������������ΪSO2
D. ����SO42-���Լ�Ϊ�����ᡢBaCl2��Һ