��Ŀ����
��13�֣�������һ����Ҫ�Ĺ�ҵԭ�ϡ�
��1��ʵ���ҿ��ö������̺�Ũ���ᷴӦ��ȡ��������Ӧ�Ļ�ѧ����ʽ�� ��
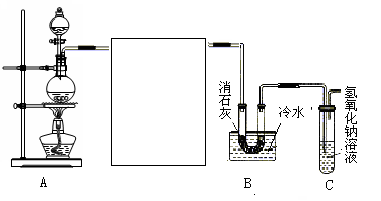
��2��������ʾ��Ca(ClO)2 +CaCl2+2H2SO4 2CaSO4+2Cl2��+2H2O��ijѧϰС�����ô�ԭ�������ͼ��ʾװ����ȡ������̽�������ʡ�
2CaSO4+2Cl2��+2H2O��ijѧϰС�����ô�ԭ�������ͼ��ʾװ����ȡ������̽�������ʡ�

���ڸ�ʵ���У��ײ��ֵ�װ���� ������ĸ����

����װ����FeCl2��Һ��Cl2��Ӧ�����ӷ���ʽ�� ��֤��FeCl2��Cl2������������ԭ��Ӧ��ʵ�鷽���� ��
�۱�װ����ͨ������Cl2�����Ƶ�ij�������г��õ�Ư�ס����������ʡ���֪̼�������ǿ�ڴ����ᣬ����з�����Ӧ�Ļ�ѧ����ʽ�� ��
�ܸ�ʵ��������Ե�ȱ�ݣ��Ľ��ķ����� ��
��3��Ϊ�ⶨƯ����Ca(ClO)2��������������С�齫2.0 gƯ�����Ƴ�250 mL��Һ��ȡ��25 mL�����������μ������ϡH2SO4������KI��Һ����ȫ��Ӧ���ٵ���0.1 mol/L Na2S2O3��Һ��2Na2S2O3+I2 ="==" Na2S4O6+2NaI��������20 mL Na2S2O3����Ư����Ca(ClO)2����������Ϊ ��
��1��ʵ���ҿ��ö������̺�Ũ���ᷴӦ��ȡ��������Ӧ�Ļ�ѧ����ʽ�� ��
��2��������ʾ��Ca(ClO)2 +CaCl2+2H2SO4
 2CaSO4+2Cl2��+2H2O��ijѧϰС�����ô�ԭ�������ͼ��ʾװ����ȡ������̽�������ʡ�
2CaSO4+2Cl2��+2H2O��ijѧϰС�����ô�ԭ�������ͼ��ʾװ����ȡ������̽�������ʡ�
���ڸ�ʵ���У��ײ��ֵ�װ���� ������ĸ����

����װ����FeCl2��Һ��Cl2��Ӧ�����ӷ���ʽ�� ��֤��FeCl2��Cl2������������ԭ��Ӧ��ʵ�鷽���� ��
�۱�װ����ͨ������Cl2�����Ƶ�ij�������г��õ�Ư�ס����������ʡ���֪̼�������ǿ�ڴ����ᣬ����з�����Ӧ�Ļ�ѧ����ʽ�� ��
�ܸ�ʵ��������Ե�ȱ�ݣ��Ľ��ķ����� ��
��3��Ϊ�ⶨƯ����Ca(ClO)2��������������С�齫2.0 gƯ�����Ƴ�250 mL��Һ��ȡ��25 mL�����������μ������ϡH2SO4������KI��Һ����ȫ��Ӧ���ٵ���0.1 mol/L Na2S2O3��Һ��2Na2S2O3+I2 ="==" Na2S4O6+2NaI��������20 mL Na2S2O3����Ư����Ca(ClO)2����������Ϊ ��
��1��MnO2 + 4HCl��Ũ�� MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O
��2���� c ��1�֣� ��2Fe2++Cl2 ="==" 2Fe3++2Cl-
ȡ������Ӧ�����Һ���ڽྻ���Թ��У������еμ�KSCN��Һ����������ɫ��Һ��֤��������Fe3+ ����FeCl2��Cl2������������ԭ��Ӧ���������÷֣�
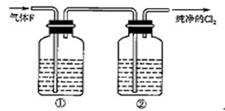
��Cl2+2Na2CO3+H2O ="==" NaCl+NaClO+2NaHCO3
���ڱ�װ�ú�����һ��ʢ������������Һ��ϴ��ƿ���������÷֣�
��3��35.75%
 MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O ��2���� c ��1�֣� ��2Fe2++Cl2 ="==" 2Fe3++2Cl-
ȡ������Ӧ�����Һ���ڽྻ���Թ��У������еμ�KSCN��Һ����������ɫ��Һ��֤��������Fe3+ ����FeCl2��Cl2������������ԭ��Ӧ���������÷֣�
��Cl2+2Na2CO3+H2O ="==" NaCl+NaClO+2NaHCO3
���ڱ�װ�ú�����һ��ʢ������������Һ��ϴ��ƿ���������÷֣�
��3��35.75%
��1������ʽΪMnO2 + 4HCl��Ũ�� MnCl2 + Cl2��+ 2H2O��
MnCl2 + Cl2��+ 2H2O��
��2���ٸ��ݷ�Ӧԭ����֪����Ӧ��Ҫ���ȣ���������Һ������Ӧ��ѡ��cװ�á�
���������������ԣ��ܰ��Ȼ��������������Ȼ���������ʽΪ2Fe2++Cl2 ="==" 2Fe3++2Cl-���������������Ȼ���������Ҫ֤��FeCl2��Cl2������������ԭ��Ӧ������ͨ��������������֤������ȡ������Ӧ�����Һ���ڽྻ���Թ��У������еμ�KSCN��Һ����������ɫ��Һ��֤��������Fe3+ ����FeCl2��Cl2������������ԭ��Ӧ��
������̼�������ǿ�ڴ����ᣬ���Է�Ӧ�в���������CO2����˷���ʽΪCl2+2Na2CO3+H2O ="==" NaCl+NaClO+2NaHCO3��
�����������ж������ڴ�����Ⱦ���Ҫβ����������Ӧ�����ڱ�װ�ú�����һ��ʢ������������Һ��ϴ��ƿ��
��3��������ƺ͵⻯�ط�Ӧ�ķ���ʽΪClO����2I����2H��=I2��Cl����H2O�����Դ�����ƺ�Na2S2O3�Ĺ�ϵʽΪCa(ClO)2��4Na2S2O3������25ml��Һ�к��д�����Ƶ����ʵ�����0.1 mol/L��0.02L��4��0.0005mol��������0.0005mol��143g/mol��0.0715g����������������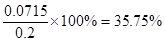 ��
��
 MnCl2 + Cl2��+ 2H2O��
MnCl2 + Cl2��+ 2H2O����2���ٸ��ݷ�Ӧԭ����֪����Ӧ��Ҫ���ȣ���������Һ������Ӧ��ѡ��cװ�á�
���������������ԣ��ܰ��Ȼ��������������Ȼ���������ʽΪ2Fe2++Cl2 ="==" 2Fe3++2Cl-���������������Ȼ���������Ҫ֤��FeCl2��Cl2������������ԭ��Ӧ������ͨ��������������֤������ȡ������Ӧ�����Һ���ڽྻ���Թ��У������еμ�KSCN��Һ����������ɫ��Һ��֤��������Fe3+ ����FeCl2��Cl2������������ԭ��Ӧ��
������̼�������ǿ�ڴ����ᣬ���Է�Ӧ�в���������CO2����˷���ʽΪCl2+2Na2CO3+H2O ="==" NaCl+NaClO+2NaHCO3��
�����������ж������ڴ�����Ⱦ���Ҫβ����������Ӧ�����ڱ�װ�ú�����һ��ʢ������������Һ��ϴ��ƿ��
��3��������ƺ͵⻯�ط�Ӧ�ķ���ʽΪClO����2I����2H��=I2��Cl����H2O�����Դ�����ƺ�Na2S2O3�Ĺ�ϵʽΪCa(ClO)2��4Na2S2O3������25ml��Һ�к��д�����Ƶ����ʵ�����0.1 mol/L��0.02L��4��0.0005mol��������0.0005mol��143g/mol��0.0715g����������������
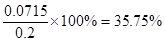 ��
��
��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ


 (NH4)2S2O8+H2����
(NH4)2S2O8+H2����