题目内容
【题目】硫酸亚铁铵(NH4)aFeb(SO4)c·dH2O又称莫尔盐,是浅绿色晶体,用硫铁矿(主要含FeS2、SiO2等)制备莫尔盐的流程如下:
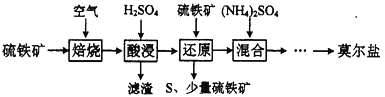
已知:“还原”时,FeS2与H2SO4不反应,Fe3+通过反应Ⅰ、Ⅱ被还原,反应Ⅰ如下:
FeS2 +14Fe3++8H2O =15Fe2++2SO42-+16H+
(1)写出“还原”时Fe3+与FeS2发生还原反应Ⅱ的离子方程式:__________________________。实验室检验“还原”已完全的方法是:__________________________。
(2)称取11.76g新制莫尔盐,溶于水配成250mL溶液。取25.00mL该溶液加入足量的BaCl2溶液,得到白色沉淀1.398g;另取25.00mL该溶液用0.0200mol/LKMnO4酸性溶液滴定,当MnO4-恰好完全被还原为Mn2+时,消耗溶液的体积为30.00mL。试确定莫尔盐的化学式(请写出计算过程)_________。
【答案】 FeS2+2Fe3+=3Fe2++2S↓ 取少量酸浸后的溶液,向其中滴加数滴KSCN溶液,如果溶液变红则“还原”未完全,反之,已完全。 原样品中的n(SO42-)=10×1.398g÷233g/mol=0.06mol
n(MnO4-)=0.0200mol/L×0.03L=0.0006 mol
由得失电子守恒可知:n(Fe2+)=5 n(MnO4-)=0.003mol 原样品中n(Fe2+)=0.03 mol
由电荷守恒可知:原样品中n(NH4+)=0. 06mol
由质量守恒:n(H2O)=0.18 mol
故化学式为:(NH4)2Fe(SO4)26H2O或(NH4)2SO4FeSO46H2O
【解析】硫铁矿(主要含FeS2、SiO2等)在空气中焙烧,得二氧化硫气体和氧化铁、二氧化硅等固体,加硫酸溶解,过滤除去二氧化硅,得硫酸铁溶液,硫酸铁溶液中再加入硫铁矿,FeS2把铁离子还原为Fe2+,同时生成S沉淀,过滤,滤渣含有S、硫铁矿,滤液中主要含有硫酸亚铁的混合溶液,向混合溶液中加入硫酸铵,经过蒸发浓缩降温结晶,过滤,洗涤,干燥可得莫尔盐。
(1)根据上面的分析可知,“还原”时,铁离子做氧化剂,如果pH过高时铁元素将沉淀导致产率降低,“还原”时FeS2把铁离子还原为Fe2+,同时生成S沉淀,所以反应Ⅱ的离子方程式为FeS2+2Fe3+=3Fe2++2S↓,实验室检验“还原”已完全的方法是取少量酸浸后的溶液,向其中滴加数滴KSCN溶液,如果溶液变红则“还原”未完全,反之,已完全,故答案为:FeS2+2Fe3+=3Fe2++2S↓; 取少量酸浸后的溶液,向其中滴加数滴KSCN溶液,如果溶液变红则“还原”未完全,反之,已完全;
(2)称取11.76g新制莫尔盐,溶于水配成250mL溶液,取25.00mL该溶液,加入足量的BaCl2溶液,得到白色沉淀硫酸钡的质量为1.398g,其物质的量为![]() =0.006mol,所以n(SO42-)=0.006mol,另取25.00mL该溶液用0.0200mol/L KMnO4酸性溶液滴定,当MnO4-恰好完全被还原为Mn2+时,消耗溶液的体积为30.00mL,根据题意,
=0.006mol,所以n(SO42-)=0.006mol,另取25.00mL该溶液用0.0200mol/L KMnO4酸性溶液滴定,当MnO4-恰好完全被还原为Mn2+时,消耗溶液的体积为30.00mL,根据题意,
5Fe2+ + MnO42-+8H+=5Fe3++Mn2++4H2O
5 1
n(Fe2+) 0.02000molL-1×0.03L,
所以n(Fe2+)=0.003mol,根据电荷守恒:n(NH4+)+2n(Fe2+)=2n(SO42-),n(NH4+)=0.006mol;250mL溶液中n(SO42-)=0.06mol,n(Fe2+)=0.03mol,n(NH4+)=0.06mol,n(H2O)= ![]() =0.18 mol,所以n(SO42-):n(Fe2+):n(NH4+):n(H2O)=0 06 mol:0 03 mol:0 06mol:0 18 mol=2:1:2:6,所以莫尔盐的化学式为(NH4)2Fe(SO4)26H2O[或(NH4)2SO4FeSO46H2O],故答案为:(NH4)2Fe(SO4)26H2O[或(NH4)2SO4FeSO46H2O]。
=0.18 mol,所以n(SO42-):n(Fe2+):n(NH4+):n(H2O)=0 06 mol:0 03 mol:0 06mol:0 18 mol=2:1:2:6,所以莫尔盐的化学式为(NH4)2Fe(SO4)26H2O[或(NH4)2SO4FeSO46H2O],故答案为:(NH4)2Fe(SO4)26H2O[或(NH4)2SO4FeSO46H2O]。