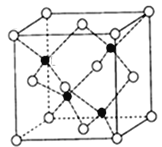
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ–ΓΟς‘ΎΩΈΆβ‘ΡΕΝ÷–ΒΟ÷ΣΘΚ«β―θΜ·Ά≠ ή»»ΜαΖ÷Ϋβ…ζ≥…―θΜ·Ά≠Θ®Ca(OH)2=CuO+XΘ©Θ§”Ύ «”ΟCuSO4»ή“Κ”κKOH»ή“ΚΖ¥”Π÷Τ»Γ«β―θΜ·Ά≠Θ§≤ΔΕ‘«β―θΜ·Ά≠Ϋχ––Φ”»»ΓΘ
(1)…ζ≥…ΈοXΒΡΜ·―ß ΫΈΣ___________ΓΘ
(2)÷Τ»Γ«β―θΜ·Ά≠ΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______________________ΓΘ
(3)–ΓΟς‘ΎΦ”»»«β―θΜ·Ά≠ ±Θ§ΖΔœ÷άΕ…ΪΙΧΧεœ»±δ≥…ΚΎ…ΪΘΜΦΧ–χΗΏΈ¬ΉΤ…’ΚΎ…ΪΙΧΧε±δ≥…Κλ…ΪΘ§Ά§ ±”–ΤχΧε≤ζ…ζΓΘΈΣ≈Σ«ε’β÷÷Κλ…ΪΙΧΧεΒΡ≥…ΖίΘ§Ϋχ––ΝΥ»γœ¬ΒΡΧΫΨΩΘΚ
Θ®≤ι‘ΡΉ ΝœΘ©CuΚΆCu2OΨυΈΣΚλ…ΪΙΧΧεΘ§«“Cu2O+H2SO4=Cu+CuSO4+H2OΓΘ
Θ®Χα≥ω≤¬œκΘ©Κλ…ΪΙΧΧε «ΘΚΔώ.Cu Δρ.Cu2O Δσ. ______________________ΓΘ
Θ®Ϋχ–– Β―ιΘ©
≤ΌΉς | œ÷œσ | Ϋα¬έ |
ΔΌ»ΓΉΤ…’ΚσΒΡΚλ…ΪΙΧΧε1.44g”Ύ ‘Ιή÷–Θ§Φ”»κΉψΝΩœΓΝρΥαΘ§Φ”»»≤Δ≥δΖ÷’πΒ¥Θ§Ψ≤÷ΟΓΘ | »ή“Κ”…Έό…Ϊ±δάΕ…ΪΘ§ΙΧΧεΦθ…ΌΓΘ | 1.44gΚλ…ΪΙΧΧε“ΜΕ®”–ΘΚ____________________ |
ΔΎΙΐ¬ΥΓΔœ¥Β”ΓΔΗ…‘οΓΘ | ΒΟΚλ…ΪΙΧΧε |
ΓΨ¥πΑΗΓΩH2O CuSO4+2KOH=Cu(OH)2![]() +K2SO4 CuΚΆCu2O Cu2O
+K2SO4 CuΚΆCu2O Cu2O
ΓΨΫβΈωΓΩ
ΗυΨί‘ΣΥΊ ΊΚψΩ…÷ΣΘ§«β―θΜ·Ά≠ ή»»Ζ÷Ϋβ…ζ≥…―θΜ·Ά≠ΚΆΥ°ΓΘ”…”ΎCuΚΆCu2OΨυΈΣΚλ…ΪΙΧΧεΘ§Ι ΆΤ≤βΚλ…ΪΙΧΧε≥…Ζ÷Ω…ΡήΈΣCu ΜρCu2OΜρΝΫ’ΏΒΡΜλΚœΈοΓΘ
Θ®1Θ©ΗυΨί‘ΣΥΊ ΊΚψΘ§Ca(OH)2=CuO+X÷–XΈΣH2OΓΘ
Θ®2Θ©”ΟCuSO4»ή“Κ”κKOH»ή“ΚΖ¥”Π…ζ≥…«β―θΜ·Ά≠ΚΆΝρΥαΦΊΘ§Μ·―ßΖΫ≥Χ ΫΈΣCuSO4+2KOH=Cu(OH)2![]() +K2SO4ΓΘ
+K2SO4ΓΘ
Θ®3Θ©”…”ΎCuΚΆCu2OΨυΈΣΚλ…ΪΙΧΧεΘ§Ι ΆΤ≤βΚλ…ΪΙΧΧε≥…Ζ÷”–»ΐ÷÷Ω…Ρή–‘ΘΚ÷Μ”–”…”ΎCuΜρ’Ώ÷Μ”–Cu2OΜρ’ΏΈΣCuΚΆCu2OΒΡΜλΚœΈοΘΜΆ≠≤ΜΡή”κœΓΝρΥαΖ¥”ΠΘ§ΒΪΚλ…ΪΙΧΧεΦ”»κœΓΝρΥαΚσΘ§»ή“Κ±δΈΣάΕ…Ϊ≤Δ«“ΙΧΧεΦθ–ΓΘ§ΥΒΟςΙΧΧε”κœΓΝρΥαΖ¥”Π…ζ≥…Ά≠άκΉ”Θ§ΖΔ…ζΖ¥”ΠCu2O+H2SO4=Cu+CuSO4+H2OΘ§Ι Κλ…ΪΙΧΧε÷–“ΜΕ®Κ§”–Cu2OΓΘ

 ΩΈΧΟ»ΪΫβΉ÷¥ ΨδΕΈΤΣ’¬œΒΝ–¥πΑΗ
ΩΈΧΟ»ΪΫβΉ÷¥ ΨδΕΈΤΣ’¬œΒΝ–¥πΑΗ ≤Ϋ≤ΫΗΏΩΎΥψΧβΩ®œΒΝ–¥πΑΗ
≤Ϋ≤ΫΗΏΩΎΥψΧβΩ®œΒΝ–¥πΑΗ ΒψΨΠ–¬ΫΧ≤Ρ»ΪΡήΫβΕΝœΒΝ–¥πΑΗ
ΒψΨΠ–¬ΫΧ≤Ρ»ΪΡήΫβΕΝœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΓΑΧβΆΦΓ± «≤ΩΖ÷‘ΣΥΊΒΡάκΉ”ΫαΙΙ Ψ“βΆΦΚΆ‘ΣΥΊ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷ΓΘ «κΜΊ¥π:
(1)ΆΦΔΌΓΔΔΎ÷– τ”Ύ“θάκΉ”ΒΡ «______(Χν–ρΚ≈)Θ§ΗΟ‘ΣΥΊΒΡ÷ Ή” ΐΈΣ______ΘΜ13Κ≈‘ΣΥΊ‘≠Ή”ΒΡΉνΆβ≤ψΒγΉ” ΐΈΣ_________ΓΘ
ΔΌ![]() ΔΎ
ΔΎ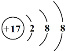
| ||||||
| ||||||
|
| |||||
(2)”…±μ÷–‘≠Ή”–ρ ΐΈΣ1ΓΔ8ΓΔ13ΒΡ‘ΣΥΊΉι≥…Έο÷ ΒΡΜ·―ß ΫΈΣ_____________ΓΘ