��Ŀ����
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
��ش�
(1)�������������ʵ���������Ӽ�����������������a(�����Һ��pH)��˵��HA��ǿ�ỹ�����________________________________________________
(2)c1________(�����������������)0.2 mol��L��1������ʵ����HA��NaOH��Һ���ǰ��HA��Һ��[A��]��NaOH��Һ��[Na��]�Ĺ�ϵ��________(������ѡ����ѡ�����)��
A��ǰ�ߴ� B�����ߴ�
C��������� D�����ж�
(3)�ӱ���ʵ�����������û����Һ������Ũ���ɴ�С��˳����__________________________�����У�[A��]��________ mol��L��1(���������Ƽ��㣬�ش�ȷֵ�������һ��Ҫ����)��
(4)����ʵ���У�HA��NaOH��Һ���ǰ[HA]________(�����������������)[NaOH]��b________(�����������������)7��
| ʵ���� | HA | NaOH | �����Һ��pH |
| �� | [HA]��0.2 mol��L��1 | [NaOH]��0.2 mol��L��1 | pH��a |
| �� | [HA]��c1 mol��L��1 | [NaOH]��0.2 mol��L��1 | pH��7 |
| �� | [HA]��0.1 mol��L��1 | [NaOH]��0.1 mol��L��1 | pH��9 |
| �� | pH��2 | pH��12 | pH��b |
��ش�
(1)�������������ʵ���������Ӽ�����������������a(�����Һ��pH)��˵��HA��ǿ�ỹ�����________________________________________________
(2)c1________(�����������������)0.2 mol��L��1������ʵ����HA��NaOH��Һ���ǰ��HA��Һ��[A��]��NaOH��Һ��[Na��]�Ĺ�ϵ��________(������ѡ����ѡ�����)��
A��ǰ�ߴ� B�����ߴ�
C��������� D�����ж�
(3)�ӱ���ʵ�����������û����Һ������Ũ���ɴ�С��˳����__________________________�����У�[A��]��________ mol��L��1(���������Ƽ��㣬�ش�ȷֵ�������һ��Ҫ����)��
(4)����ʵ���У�HA��NaOH��Һ���ǰ[HA]________(�����������������)[NaOH]��b________(�����������������)7��
(1)��a��7��HA��ǿ�ᣬ��a>7��HA������
(2)����B
(3)[Na��]��[A��]��[OH��]��[H��]��0.05��1��10��5��1��10��9
(4)������
(2)����B
(3)[Na��]��[A��]��[OH��]��[H��]��0.05��1��10��5��1��10��9
(4)������
(1)������HA��NaOHǡ����ȫ��Ӧ����NaA��H2O������Һ�����ԣ�˵��A����ˮ�⣬HA��ǿ�ᣬ����Һ�ʼ��ԣ�˵��A��ˮ�⣬HA�����(2)��������Һ�����ԣ�������Һ���ܳʼ��ԣ�����[HA]���ڼ��е�Ũ�ȣ�������HA����ȫ���룬����[A��]ҪԶС��NaOH��Һ��[Na��]��(3)��������Һ�ʼ��ԣ�[OH��]��[H��]���ɵ���غ�[Na��]��[H��]��[A��]��[OH��]֪[Na��]��[A��]����������Ũ���ɴ�С��˳����[Na��]��[A��]��[OH��]��[H��]��[A��]��[Na��]��[H��]��[OH��]��(0.05��1��10��9��1��10��5)mol��L��1��(4)HA��pH��2����[H��]��10��2mol��L��1��[HA]��10��2mol��L��1��NaOH��Һ��pH��12����[OH��]��10��2mol��L��1��[NaOH]��10��2mol��L��1������Ϻ�HA��������Һ�����ԣ�pH��7��

��ϰ��ϵ�д�
�����Ŀ
 ��I����NO
��I����NO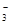
 ��HCO
��HCO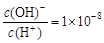 �� ��0.01 mol��L��1HA��Һ��c(H+)=1��10��4 mol��L��1
�� ��0.01 mol��L��1HA��Һ��c(H+)=1��10��4 mol��L��1 H+ + OH��
H+ + OH��