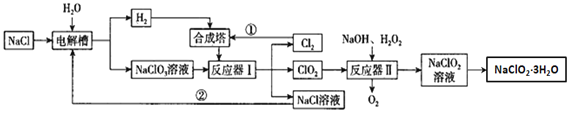
��Ŀ����
����Ŀ����.��֪25�棬NH3��H2O��Kb=1.8��105��H2SO3��Ka1=1.3��102��Ka2=6.2��108������ˮ��Ũ��Ϊ2.0 mol��L1����Һ�е�c(OH)=_______mol��L1��0.1 mol��L1��(NH4)2SO3��Һ��______������ԡ��������ԡ������ԡ�����
��. ijѧ����0.200mol/L�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
��������ˮϴ�Ӽ�ʽ�ζ��ܣ���ע��NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�۵���Һ������0����0���̶������£������¶���
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ
���ñ�Һ�ζ����յ㣬���µζ���Һ�����
��ش�
��1�����ϲ����д������______ �����ţ���
��2���ζ��յ������Ϊ____________________________________________��
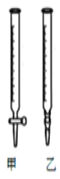
��3���ñ�NaOH��Һ�ζ�ʱ��Ӧ����NaOH��Һע��______ ��(��ͼ��ѡ��ס����ҡ�)��
��4�����в���������ʵ����ƫ�����______![]() ����
����![]() ��
��
A������ƿװҺǰ��������������ˮ
B���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������
C���ζ��յ㸩�Ӷ���
���𰸡�6.0��10-3 ���� �� �������һ������������Һ����Һǡ������ɫ��ɷۺ�ɫ���Ұ�����ڲ���ɫ �� B
��������
��.��֪25�棬NH3��H2O��Kb=1.8��105��H2SO3��Ka1=1.3��102��Ka2=6.2��108������ˮ��Ũ��Ϊ2.0 mol��L1����Һ�е�c(OH)=![]() mol��L1����ΪKb=1.8��105��Ka2=6.2��108��Kb��Ka2������0.1 mol��L1��(NH4)2SO3��Һ�Լ��ԡ�
mol��L1����ΪKb=1.8��105��Ka2=6.2��108��Kb��Ka2������0.1 mol��L1��(NH4)2SO3��Һ�Լ��ԡ�
��.��������ˮϴ�Ӽ�ʽ�ζ��ܣ�û���ô�ʢװ�ļ�Һ��ϴ��������Ũ��ƫС���ٴ���
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ�壬����ȷ��
�۵���Һ������0����0���̶������£������¶���������ȷ��
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ������ȷ��
���ñ�Һ�ζ����յ㣬���µζ���Һ�����������ȷ��
��1�����ϲ����д�����Ǣ١���Ϊ���٣�
��2���ζ��յ������Ϊ�������һ������������Һ����Һǡ������ɫ��ɷۺ�ɫ���Ұ�����ڲ���ɫ��
��3��ͼ�У���Ϊ��ʽ�ζ��ܣ���Ϊ��ʽ�ζ��ܡ��ñ�NaOH��Һ�ζ�ʱ��Ӧ����NaOH��Һע�����С�
��4��A������ƿװҺǰ��������������ˮ����Һ�����ʵ������䣬��Ӱ��ζ������
B���ζ�ǰ���ζ��ܼ��������ݣ��ζ��������ݣ���ȡ�����ƫ�����ռ������Ũ��ƫ�ߣ�
C���ζ��յ㸩�Ӷ��������������ñ�Һ�����ƫС��������Ĵ���Һ��Ũ��ƫС��
��ѡB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�