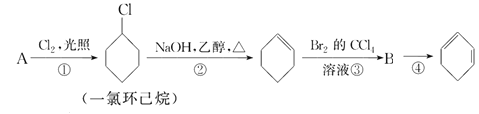
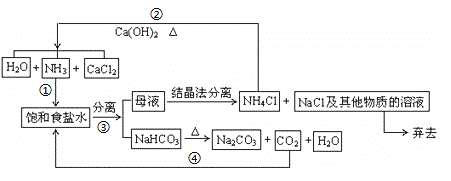
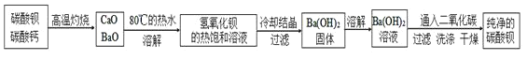
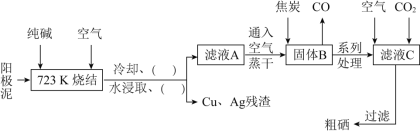��Ŀ����
����Ŀ��I.д�����з�Ӧ���Ȼ�ѧ����ʽ��
��1��CuCl(s)��O2��Ӧ����CuCl2(s)��һ�ֺ�ɫ���塣��25 ����101 kPa�£���֪�÷�Ӧÿ����1 mol CuCl(s)������44.4 kJ���÷�Ӧ���Ȼ�ѧ����ʽ��_____________________��
��2����1.01��105 Paʱ��16 g S�����������������г��ȼ�����ɶ������ų�148.5 kJ����������S����ȼ���ȵ��Ȼ�ѧ����ʽΪ________________________��
II.�о�NOx��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��3��������CO��SO2�̵�����Ⱦ��һ�ַ����ǽ����ڴ���������ת��Ϊ����S���塣��֪��
��CO(g)��![]() O2(g)=CO2(g) ��H����283.0 kJ��mol��1
O2(g)=CO2(g) ��H����283.0 kJ��mol��1
��S(s)��O2(g)=SO2(g)�� ��H����296.0 kJ��mol��1
�˷�Ӧ���Ȼ�ѧ����ʽ��_____________________��
��4��������������ɹ⻯ѧ�����ͳ�������ĵ���Ҫ���塣��֪��
CO(g)��NO2(g)=NO(g)��CO2(g) ��H����a kJ��mol��1(a>0)
2CO(g)��2NO(g)=N2(g)��2CO2(g) ��H����b kJ��mol��1(b>0)
���ñ�״����3.36 L CO��ԭNO2��N2(CO��ȫ��Ӧ)������������ת�Ƶ��ӵ����ʵ���Ϊ________mol���ų�������Ϊ______________kJ(�ú���a��b�Ĵ���ʽ��ʾ)��
���𰸡�4CuCl(s)��O2(g)=2CuCl2(s)��2CuO(s) ��H����177.6 kJ��mol��1 S(s)+O2(g)SO2(g)����H=-297 kJ��mol-1 2CO(g)��SO2(g)= S(s)��2CO2(g) ��H����270 kJ��mol��1 0.3 ![]()
��������
I.��1����ɫ����Ϊ����ͭ������1 mol CuCl(s)������44.4 kJ������4 mol CuCl(s)������177.6 kJ���ݴ���д�Ȼ�ѧ����ʽ��
��2��16 g ����0.5mol��S�����������������г��ȼ�����ɶ���������148.5 kJ�� 1molS�����������������г��ȼ�����ɶ���������297 kJ���ݴ���д�Ȼ�ѧ����ʽ��
II.��3����4�����ø�˹���ɽ��м��㣬����д��ȷ���Ȼ�ѧ����ʽ��
I.��1����ɫ����Ϊ����ͭ������1 mol CuCl(s)������44.4 kJ������4 mol CuCl(s)������177.6 kJ���Ȼ�ѧ����ʽΪ��4CuCl(s)��O2(g)=2CuCl2(s)��2CuO(s) ��H����177.6 kJ��mol��1��
��2��16 g ����0.5mol��S�����������������г��ȼ�����ɶ���������148.5 kJ�� 1molS�����������������г��ȼ�����ɶ���������297 kJ���Ȼ�ѧ����ʽΪ��S(s)+O2(g)SO2(g)����H=-297 kJ��mol-1��
II.��3�����ݸ�˹������2����-�ڵ��Ȼ�ѧ����ʽΪ��2CO(g)��SO2(g)= S(s)��2CO2(g) ��H����270 kJ��mol��1��
��4����֪����CO(g)��NO2(g)=NO(g)��CO2(g) ��H����a kJ��mol��1(a>0)
��2CO(g)��2NO(g)=N2(g)��2CO2(g) ��H����b kJ��mol��1(b>0)
���ݸ�˹������2����+�ڵã�4CO(g)��2NO2(g)= N2(g)��4CO2(g) ��H����(2a +b��kJ��mol��1��4molCO ��ԭNO2��N2������ת��8mol���ų�������Ϊ(2a +b��kJ���ñ�״����3.36 L(��0.15mol) CO��ԭNO2��N2������ת��0.3mol���ų�������Ϊ![]() kJ��
kJ��

 �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�����Ŀ��I. ��50 mL 0.50 mol��L��1������50 mL 0.55 mol��L��1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺

��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ���������________��
��2�������60 mL 0.50 mol��L��1������50 mL 0.55 mol��L��1NaOH��Һ���з�Ӧ��������ʵ����ȣ������к���________(��������������������)��
��3��ʵ��ʱ�������ἰNaOH��Һ�������Ϊ50 mL������Һ�ܶȾ�Ϊ1 g��mL��1��������Һ�ı�����c��4.18 J��g��1������1��ʵ����ʼ�¶�Ϊt1������ֹ�¶�Ϊt2�������ƶ��к��ȵļ���ʽ��H��________��
��4������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��________(����ƫ��������ƫС��������Ӱ����)��
II.ij�о���ѧϰС������H2C2O4��Һ������KMnO4��Һ�ķ�Ӧ̽������������ĸı�Ի�ѧ��Ӧ���ʵ�Ӱ����������������ʵ�飺
ʵ����� | ʵ���¶�/K | �й����� | ��Һ��ɫ������ɫ����ʱ��/s | ||||
����KMnO4��Һ | H2C2O4��Һ | H2O | |||||
V/mL | c/ mol��L��1 | VmL | c/ mol��L��1 | V/mL | |||
A | 293 | 2 | 0.02 | 4 | 0.1 | 0 | t1 |
B | T1 | 2 | 0.02 | 3 | 0.1 | V1 | 8 |
C | 313 | 2 | 0.02 | V2 | 0.1 | 1 | t2 |
��1��ͨ��ʵ��A��B����̽����________(���ⲿ����)�ĸı�Ի�ѧ��Ӧ���ʵ�Ӱ�죬����V1��________��T1��________��ͨ��ʵ��________(��ʵ�����)��̽�����¶ȱ仯�Ի�ѧ��Ӧ���ʵ�Ӱ�죬����V2��________��
��2����t1��8�����ɴ�ʵ����Եó��Ľ�����________________________________