��Ŀ����
(6��)��֪A��B��C��D�ֱ���AlCl3��BaCl2��FeSO4��NaOH���ֻ������е�һ�֣����ǵ�ˮ��Һ֮���һЩ��Ӧ�������£�
�� A + B����ɫ����������ϡ���ᣬ�������ܽ�
�� B + D����ɫ�������ڿ����з��ã������ɰ�ɫת��Ϊ���ɫ
�� C + D����ɫ��������������D��Һ����ɫ��������ʧ
�����ƶ�A B C D ����ѧʽ����
��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��ʵ����г����ɰ�ɫת��Ϊ���ɫ�Ļ�ѧ����ʽ ��
��C��Һ��D��Һ��Ӧ�����ɵİ�ɫ�����ܽ���D��Һ�е����ӷ���ʽ ��
��6�֣�����1�ֹ�6�֣���1��A BaCl2 B FeSO4 C AlCl3 D NaOH
��2�� ��4Fe(OH)2+O2+2H2O=4Fe(OH)3 ��Al(OH)3 + OH- ="=" AlO2-+ 2H2O
���������������ɫ����������ϡ�����Ӧ�������ᱵ����˵��A��B���Ȼ������������������ݷ�Ӧ��ֻ�������֪�����ɫ��������������������Bһ����������������A���Ȼ�����D���������ƣ����C���Ȼ�����
���㣺�������ʵļ���ͼ���
�����������Ǹ߿�ֻ�ij������ͣ��ѶȲ������ڻ���������Ŀ��顣����ѧ����Ҫ��ȷ�������ʵļ���ʱ��Ҫ�������ʵ��������ʺ�������Ӧ��ѡ���ʵ����Լ��ͷ�����ȷ�۲췴Ӧ�е�������������ɫ�ı仯�����������ɺ��ܽ⡢����IJ�������ζ���������ɫ�ȣ������жϡ���������֤���ɡ�

��16�֡�ÿ�ո�2�֣���֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ������������������A��Cԭ�ӵ�L����2��δ�ɶԵ��ӡ�D��Eͬ���壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��F3������M��3d�������Ϊ����״̬�����������������ش��������⣺������ʱ��������Ӧ��Ԫ�ط��ű�ʾ��
��1��д��A��B��C����Ԫ�ص縺���ɴ�С��˳�� ��
��2����A��B��C��������Ԫ����ɵ�ij�����Ӿ��壬1mol�þ��庬����λ��2mol���þ���Ļ�ѧʽ�� ��
��3��F��Mn���̣���Ԫ�صIJ��ֵ��������������±���Ԥ��a b������ڡ�����С�ڡ��������ڡ���
������
|
Ԫ�� |
Mn |
F |
|
|
������ �� |
I1 |
717 |
759 |
|
I2 |
1509 |
1561 |
|
|
I3 |
a |
b |
��4��AC2������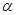 ����
���� ����Ŀ��Ϊ
��
����Ŀ��Ϊ
��
AC2������Aԭ�ӵ��ӻ���ʽ�� ��
��5��H2S��CԪ�ص��⻯�����ʽΪH2C2������Ҫ�������ʱȽ����£�
|
|
�۵�/K |
�е�/K |
��״��ʱ��ˮ�е��ܽ�� |
|
H2S |
187 |
202 |
2.6 |
|
H2C2 |
272 |
423 |
������Ȼ��� |
H2S��H2C2����Է�������������ͬ����������������ʲ������Ҫԭ���ǣ�
���۵㡢�е�������Ҫԭ��
����ˮ�е��ܽ�Ȳ������Ҫԭ��
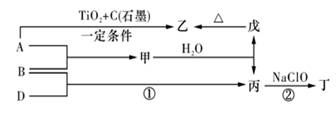
 ��
��