��Ŀ����
Q��W��X��Y��Z���ֶ�����Ԫ�أ�ԭ��������������Q��Ԫ�����ڱ���ԭ�Ӱ뾶��С��WԪ���������������۴�����Ϊ0��Y��Qͬ���壻X��Z�ֱ��ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�ء���ش��������⣺
��1��X��Z�����Ӱ뾶�ϴ��� �������ӷ��ű�ʾ����
��2����������Ԫ���е���������ɵĻ�����ס��ҡ���������ˮ��Һ��������ת����ϵ�� �����б�������ˮ�����Ե����壬����ǿ�
�����б�������ˮ�����Ե����壬����ǿ�
�����ҳ���Ϊ���Ƹ��ķ��ͷۣ����Һ��еĻ�ѧ�������� ������Һ��������Ũ��֮�� ������Ũ��֮�ͣ����������������������
�����������������Һ������ı������ҵ����ӷ���ʽΪ�� ��
��1��O2����2�֣�
��2�������Ӽ������ۼ���2�֣� ����2�֣�
��[Al(OH)4]��+CO2��Al(OH)3��+ HCO3����3�֣�
�������������Q��Ԫ�����ڱ���ԭ�Ӱ뾶��С��QΪHԪ�أ�X��Z�ֱ��ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�أ�XΪOԪ�ء�ZΪAlԪ�أ�WԪ���������������۴�����Ϊ0��WΪ��A��Ԫ�أ�����ԭ������������������WΪCԪ�أ�Y��Qͬ���壬��YΪNaԪ�ء�
��1�������Ų���ͬ�����ӣ�ԭ������Խ�����Ӱ뾶ԽС������O2?�뾶����Al3+�뾶��
��2����������ˮ�����Ե����壬����ǿ����ΪCO2,����ΪNaOH��
�����ҳ���Ϊ���Ƹ��ķ��ͷۣ�����ΪNaHCO3�����еĻ�ѧ���У����Ӽ������ۼ���������ΪH+��Na+��������ΪHCO3?��OH?��CO32?�����ݵ���غ�ɵã�c��H+��+c��Na+��=c��HCO3?��+c��OH?��+2c��CO32?��������������Ũ��֮��>������Ũ��֮�͡�
�����������������ΪAl(OH)3������Һ������ı������ҵķ�ӦΪNa[Al(OH)4]��CO2��Ӧ����Al(OH)3������NaHCO3�����ӷ���ʽΪ��[Al(OH)4]��+CO2��Al(OH)3��+ HCO3����
���㣺���⿼��Ԫ�������ʵ��ƶϡ����Ӱ뾶�Ƚϡ���ѧ�����жϡ�����Ũ�ȱȽϡ����ӷ���ʽ����д��

 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�������Ԫ�����ڱ���һ���֣�����Ԫ�آ٣����ڱ��е�λ�ã����û�ѧ����ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | | �� | |
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ(Ԫ�ط���)________________________��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����(�ѧʽ)________ ��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����Ļ�ѧʽ��_______________��
��4���ɢں͢���ɵĻ�������ݵ�ͬ������������Ԫ�صĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ:_______��
��5��������ݵ�����������ˮ���ﷴӦ�����ӷ���ʽΪ ��
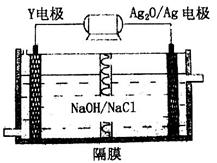
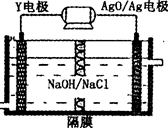
��6�����â٢��������л�����Ϊȼ�ϵ�ص�ԭ�ϣ���д���ڼ��Խ�����ȼ�ϵ�ظ����ĵ缫��Ӧʽ: ��
��7��ȼú�����еĺ��е������NOx����������̼�����壬�������з�����ȼú����������������ʱ�������ü������ԭ�������
�磺CH4(g)��4NO2(g)=4NO(g)��CO2(g)��2H2O(g) �� ��H=��574 kJ��mol��1
CH4(g)��4NO(g)=2N2(g)��CO2(g)��2H2O(g) �� ��H=��1160 kJ��mol��1
��CH4(g)��NO2(g)��ԭΪN2(g)�ȵ��Ȼ�ѧ����ʽΪ ��
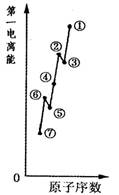
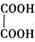 �������ƾ����д��� ����ͼ��Ԫ�ش��ţ���
�������ƾ����д��� ����ͼ��Ԫ�ش��ţ���