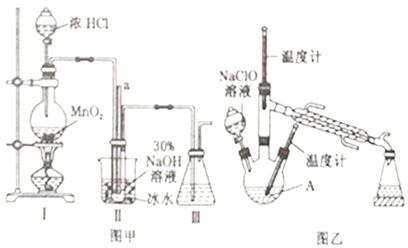
��Ŀ����
����Ŀ����֦���ۺ��������ҽҩ������繦�ܲ��ϵ������й㷺��Ӧ�á��л�������G��3��5-���屽�����������Ǻϳ���֦���ۺ������Ҫԭ�ϣ���A(�ױ�)�Ʊ�H��һ�ֺϳ�·�����£�
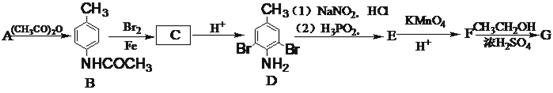
�ش��������⣺
��1��E�Ļ�ѧ����Ϊ__________��
��2��C�Ľṹ��ʽΪ____________��F�еĹ�����������__________��
��3��A����B�ķ�Ӧ����Ϊ________��
��4����F����G�Ļ�ѧ����ʽΪ___________________________________��
��5��G��ͬ���칹������ͬʱ�������������Ĺ���________�֣����������칹����
����ֱ���뱽��������
�ڱ���ֻ��3��ȡ������
�۴������������š�
��6��д�����ұ�Ϊԭ���Ʊ�������![]() �ĺϳ�·��____________�������Լ���ѡ����
�ĺϳ�·��____________�������Լ���ѡ����
���𰸡� 3��5-����ױ� 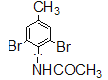 �Ȼ� ��ԭ�� ȡ����Ӧ
�Ȼ� ��ԭ�� ȡ����Ӧ 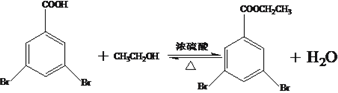 35��
35�� 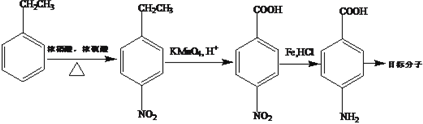
�����������������������ͼ��֪A��BΪ�����е���ԭ�ӱ�![]() ȡ����B��CΪ�����Ӧ���ٸ���D�Ľṹ�ص�ȷ��C����ԭ�ӵ�ȡ��λ�ã�����C�Ľṹ��ʽΪ��
ȡ����B��CΪ�����Ӧ���ٸ���D�Ľṹ�ص�ȷ��C����ԭ�ӵ�ȡ��λ�ã�����C�Ľṹ��ʽΪ��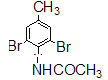 ���������ʲ��������������������ղ���G������3��5-���屽���������������Ʋ�F��GΪ������Ӧ������F��
���������ʲ��������������������ղ���G������3��5-���屽���������������Ʋ�F��GΪ������Ӧ������F��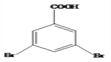 ���ٸ���E��F�������Ǹ����������֪�ⲽΪ������������Ӧ��E��
���ٸ���E��F�������Ǹ����������֪�ⲽΪ������������Ӧ��E��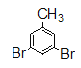 ������ȷ��D��EΪ���������뷴Ӧ��
������ȷ��D��EΪ���������뷴Ӧ��
�������������Ϸ�������1�� �Ļ�ѧ����Ϊ3��5-����ױ���
�Ļ�ѧ����Ϊ3��5-����ױ���
��2��C�Ľṹ��ʽΪ ��F��
��F�� ���������������Ȼ�����ԭ������3��A��BΪ�����е���ԭ�ӱ�
���������������Ȼ�����ԭ������3��A��BΪ�����е���ԭ�ӱ�![]() ȡ������Ӧ����Ϊȡ����Ӧ����4��
ȡ������Ӧ����Ϊȡ����Ӧ����4�� ���Ҵ�����3��5-���屽���������Ļ�ѧ����ʽΪ
���Ҵ�����3��5-���屽���������Ļ�ѧ����ʽΪ ����5������ֱ���뱽���������ڱ���ֻ��3��ȡ�������۴������������š�����������
����5������ֱ���뱽���������ڱ���ֻ��3��ȡ�������۴������������š�����������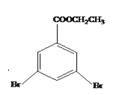 ��ͬ���칹��������ȡ�����ֱ���2����ԭ�ӡ�һ����-COOCH2CH3��ʱ����6�ֽṹ�� ����ȡ�����ֱ���2����ԭ�ӡ�һ����-CH2COOCH3��ʱ����6�ֽṹ������ȡ�����ֱ���2����ԭ�ӡ�һ����-CH2CH2OOCH��ʱ����6�ֽṹ������ȡ�����ֱ���2����ԭ�ӡ�һ����-CH2OOCCH3��ʱ����6�ֽṹ������ȡ�����ֱ���2����ԭ�ӡ�һ����-OOCCH2CH3��ʱ����6�ֽṹ������ȡ�����ֱ���2����ԭ�ӡ�һ����-CH(CH3)OOCH��ʱ����6�ֽṹ����36�ֽṹ��ȥ������������35��ͬ���칹�塣��6���ұ�����������Ӧ���ɶ������ұ����������ұ�����Ϊ�����������ᣬ�����������ỹԭΪ���������ᣬ���������ᷢ�����۷�Ӧ����
��ͬ���칹��������ȡ�����ֱ���2����ԭ�ӡ�һ����-COOCH2CH3��ʱ����6�ֽṹ�� ����ȡ�����ֱ���2����ԭ�ӡ�һ����-CH2COOCH3��ʱ����6�ֽṹ������ȡ�����ֱ���2����ԭ�ӡ�һ����-CH2CH2OOCH��ʱ����6�ֽṹ������ȡ�����ֱ���2����ԭ�ӡ�һ����-CH2OOCCH3��ʱ����6�ֽṹ������ȡ�����ֱ���2����ԭ�ӡ�һ����-OOCCH2CH3��ʱ����6�ֽṹ������ȡ�����ֱ���2����ԭ�ӡ�һ����-CH(CH3)OOCH��ʱ����6�ֽṹ����36�ֽṹ��ȥ������������35��ͬ���칹�塣��6���ұ�����������Ӧ���ɶ������ұ����������ұ�����Ϊ�����������ᣬ�����������ỹԭΪ���������ᣬ���������ᷢ�����۷�Ӧ����![]() ���ϳ�·����
���ϳ�·���� ��
��

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�