��Ŀ����
2��ijͬѧ�ڳ������������ʵ������̽��Na2S203�Ļ�ѧ���ʣ�pH=8$\stackrel{��}{\underset{\;��}{pH��ֽ}}$Na2S2O3��Һ$��_{ͬʱ�ӹ���BaCl_{2}��Һ}^{�ڼ���������ˮ}$��ɫ����B��
����˵����ȷ���ǣ�������
| A�� | ʵ���˵��Na2S2O3��Һ��ˮ�����c��OH-��=10-8mol/L | |
| B�� | Na2S2O3��ҺPH=8��ԭ�������ӷ���ʽ��ʾΪS2O32-+2H2O�TH2S2O3+2OH- | |
| C�� | ���ɵij���BaSO3��BaSO4��Ҫ��һ��ȷ�ϻ����ټ���ϡ������֤ | |
| D�� | ʵ���˵��Na2S2O3���л�ԭ�� |
���� A��������ʵ��ٲⶨ��ҺPH=8��˵����Һ�ʼ��ԣ�H2S2O3��һ�����
B��Na2S2O3��ҺPH=8��Һ�ʼ��ԣ���������������ˮ���ԭ��������������ˮ��ֲ����У�
C��ʵ��ڷ�����Ӧ�������������� ���������Ϊ�����ƣ���ϱ������������ᱵ������
D��ʵ��ڷ�����Ӧ�����������������������Ϊ�����ƣ���Ԫ�ػ��ϼ۷����仯��Ԫ�ػ��ϼ�����˵��Na2S2O3���л�ԭ�ԣ�
��� �⣺������ʵ��ٲⶨ��ҺpH=8��˵����Һ�ʼ��ԣ�H2S2O3��һ�����ᣬ���ڶ�Ժ����ֲ����룻
A����Na2S2O3��ҺpH=8����Һ�ʼ��ԣ�������������ˮ���Լ��ԣ�ˮ�����c��OH-��=10-6mol/L����A����
B��Na2S2O3��ҺpH=8����Һ�ʼ��ԣ�������������ˮ���Լ��ԣ�֤��H2S2O3��һ�����ᣬNa2S2O3ˮ��ֲ����У���Ӧ�����ӷ���ʽΪS2O32-+H2O?HS2O3-+OH-��HS2O3-+H2O?H2S2O3+OH-����B����
C��ʵ��ڷ�����Ӧ�����������������������Ϊ�����ƣ�˵��Na2S2O3���л�ԭ�ԣ������ƽ�ϱ������������ᱵ��������Ӧ�����ӷ���ʽ��S2O32-+5H2O+4Cl2+Ba2+=2BaSO4��+8Cl-+10H+����C����
D��ʵ��ڷ�����Ӧ�����������������������Ϊ�����ƣ���Ԫ�ػ��ϼ�����ʧ���ӷ���������Ӧ��˵��Na2S2O3���л�ԭ�ԣ���D��ȷ��
��ѡD��
���� ���⿼������Һ����Ժͷ�Ӧ�����жϣ�����ˮ��Ӧ�ã����ӷ���ʽ����д�ȣ���Ŀ�漰��֪ʶ��϶࣬�����ڿ���ѧ���Ի���֪ʶ��Ӧ�����������ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

| A�� | CH3-CH=CH2��CH2=CH2�����ʽ��ͬ | |
| B�� | CH��CH��C6H6��̼����ͬ | |
| C�� | ��Ȳ�ͱ���Ϊͬϵ�� | |
| D�� | �����顢�����顢������ķе����� |
| A�� | ����������Һ������ķ�ӦOH-+H+�TH2O | |
| B�� | �����ʯ��ˮ��ϡ���ᷴӦ Ca��OH��2+2H+�TCa2++2H2O | |
| C�� | ͭƬ������������Һ�� Cu+Ag+�TCu2++Ag | |
| D�� | ̼�������ϡ������ CaCO3+2H+�TCa2++H2O+CO2�� |
| A�� | C2H5OH | B�� | H2O | C�� | CH3COOH | D�� | C6H12 |
| A�� | ij�л���ȼ��ֻ����CO2��H2O���Ҷ������ʵ�����ȣ�����л�������ΪCnH2n | |
| B�� | ��ͬ���ʵ�����������ȫȼ�գ����ɵ�CO2Խ�࣬˵������C�İٷֺ���Խ�� | |
| C�� | ij��̬��CxHy������O2ǡ����ȫ��Ӧ�������Ӧǰ������������䣨�¶ȣ�100�棩����y=4����������٣���y��4 | |
| D�� | ��ͬ������������ȫȼ�գ�����O2Խ�࣬����H�İٷֺ���Խ�� |
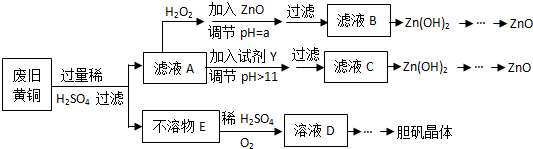
��֪��Zn���������������Al����������������ƣ�pH��11ʱZn��OH��2������NaOH��Һ����[Zn��OH��4]2-�±��г��˼������������������������pH����ʼ������pH����������Ũ��Ϊ1.0mol•L-1���㣩��
| Fe3+ | Fe2+ | Zn2+ | |
| ��ʼ������pH | 1.1 | 5.8 | 5.9 |
| ������ȫ��pH | 3.0 | 8.8 | 8.9 |
��1������ZnO����pH=a��Ŀ����ʹ��Һ�е�Fe3+��ȫ������Zn2+����������a�ķ�Χ��3.0��a��5.9��
��2���ɲ�����E������ҺD�Ļ�ѧ����ʽΪ2Cu+O2+2H2SO4=2CuSO4+2H2O��
��3����ҺA�м���H2O2��Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��
��4������ҺD�Ƶ��������������Ҫ��������������Ũ������ȴ�ᾧ�����ˣ�
��5�������Լ�����ΪY�Լ�����B��
A��ZnO B��NaOH C��Na2CO3D��ZnSO4
������ҺC����μ�������ֱ����������������������Ȳ�����ɫ����������ܽ⣮

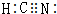 ��HCN��CԪ�صĻ��ϼ�Ϊ+2�ۣ�
��HCN��CԪ�صĻ��ϼ�Ϊ+2�ۣ�