��Ŀ����
������60%��þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£��ٰѱ����Ƴ�ʯ���顡���ں�ˮ�м���ʯ���飬���ˣ�ϴ�ӳ�����۽������������ᷴӦ���ᾧ�����ˡ������Ȼ����������и��ᄃ�塡�ݵ��������������������˵������ȷ���� (����)��
| A��þԪ����Ԫ�����ڱ���λ�ڵ�3���ڣ���A�� |
| B����ϴ��Һ�еμ�̼������Һ�ɼ�������Ƿ�ϴ�Ӹɾ� |
| C�����Ȼ����������и��ᄃ���Ŀ��������MgCl2ˮ�� |
| D�������Ҳ���Բ��õ��þ���ˮ��Һ�ķ��� |
D
D���в����ķ�Ӧ��ѧ����ʽ��MgCl2��2H2O Mg(OH)2����
Mg(OH)2����
H2����Cl2���������MgCl2��Һ�ò���þ��
 Mg(OH)2����
Mg(OH)2����H2����Cl2���������MgCl2��Һ�ò���þ��

��ϰ��ϵ�д�
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
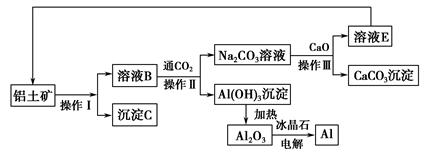
�����Ŀ
 2Na +Cl2��
2Na +Cl2��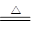 Mg+ H2O
Mg+ H2O  3 Fe +4 CO2
3 Fe +4 CO2
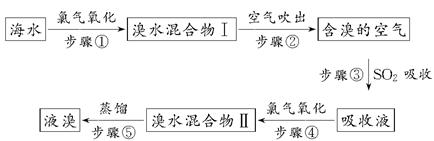

 2CuI(��ɫ)����I2��I2��2S2O32��
2CuI(��ɫ)����I2��I2��2S2O32��