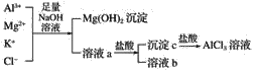
ĖâÄŋÄÚČÝ
ĄūĖâÄŋĄŋČįÍžĢŽÔÚŨóĘÔđÜÖÐÏČžÓČë2 mL 95%ĩÄŌŌīžĢŽēĒÔÚŌĄķŊÏÂŧšŧšžÓČë3 mLÅĻÁōËáĢŽÔŲžÓČë2 mLŌŌËáĢŽģä·ÖŌĄÔČĄĢÔÚÓŌĘÔđÜÖОÓČë5 mLąĨšÍNa2CO3ČÜŌšĄĢ°īÍžÁŽ―ÓšÃŨ°ÖÃĢŽÓÃūÆūŦĩÆķÔŨóĘÔđÜÐĄŧðžÓČČ3ĄŦ5 minšóĢŽļÄÓÃīóŧðžÓČČĢŽĩąđÛēėĩ―ÓŌĘÔđÜÖÐÓÐÃũÏÔÏÖÏóĘąÍĢÖđĘĩŅéĄĢ
(1)ÐīģöŨóĘÔđÜÖÐÖũŌŠ·īÓĶĩÄŧŊŅ§·―ģĖĘ―Ģš_________________________________ĄĢ
(2)žÓČëÅĻÁōËáĩÄŨũÓÃĢš_________________ĄĢ
(3)·īÓĶŋŠĘžĘąÓÃūÆūŦĩÆķÔŨóĘÔđÜÐĄŧðžÓČČĩÄÔŌōĘĮ____________________________(ŌŅÖŠŌŌËáŌŌõĨĩÄ·ÐĩãΊ77 ĄæĢŧŌŌīžĩÄ·ÐĩãΊ78.5 ĄæĢŧŌŌËáĩÄ·ÐĩãΊ117.9 Ąæ)ĢŧšóļÄÓÃīóŧðžÓČČĩÄÄŋĩÄĘĮ______ĄĢ
(4)·ÖĀëÓŌĘÔđÜÖÐËųĩÃŌŌËáŌŌõĨšÍąĨšÍNa2CO3ČÜŌšĩÄēŲŨũΊ________(ÖŧĖîÃûģÆ)ĢŽËųÐčÖũŌŠŌĮÆũΊ__________ĄĢ
Ąūīð°ļĄŋCH3COOHĢŦCH3CH2OH![]() CH3COOCH2CH3ĢŦH2O īßŧŊžÁĄĒÎüËŪžÁ žÓŋė·īÓĶËŲÂĘĢŽÍŽĘąÓÖ·ĀÖđ·īÓĶÎïÎīĀīĩÞ°·īÓĶķøŧÓ·ĒĩÄËðʧ ÕôģöÉúģÉĩÄŌŌËáŌŌõĨĢŽĘđŋÉÄæ·īÓĶÏōÓŌ―øÐÐ ·ÖŌš ·ÖŌšÂĐķ·
CH3COOCH2CH3ĢŦH2O īßŧŊžÁĄĒÎüËŪžÁ žÓŋė·īÓĶËŲÂĘĢŽÍŽĘąÓÖ·ĀÖđ·īÓĶÎïÎīĀīĩÞ°·īÓĶķøŧÓ·ĒĩÄËðʧ ÕôģöÉúģÉĩÄŌŌËáŌŌõĨĢŽĘđŋÉÄæ·īÓĶÏōÓŌ―øÐÐ ·ÖŌš ·ÖŌšÂĐķ·
Ąū―âÎöĄŋ
ĢĻ1ĢĐŌŌËáÓëŌŌīžÔÚÅĻÁōËáŨöīßŧŊžÁĄĒÎüËŪžÁĖõžþÏÂÉúģÉŌŌËáŌŌõĨšÍËŪĢŧ
ĢĻ2ĢĐÅĻÁōËáūßÓÐÎüËŪÐÔĢŽÔÚŌŌËáÓëŌŌīž·ĒÉúõĨŧŊ·īÓĶĘąŨũīßŧŊžÁšÍÎüËŪžÁĢŧ
ĢĻ3ĢĐŌĀūÝÉýļßÎÂķČŋÉŌÔžÓŋė·īÓĶËŲÂĘĢŽ―ášÏŌŌīžĄĒŌŌËáĩÄŧÓ·ĒÐÔ―âīðĢŧ
ĢĻ4ĢĐ·ÖĀëŧĨēŧÏāČÜĩÄÁ―ÖÖŌšĖåĢŽÓĶŅĄÔņ·ÖŌšēŲŨũĢŽÖũŌŠÓÃĩ―ŌĮÆũ·ÖŌšÂĐķ·Ģŧ
ĢĻ1ĢĐŌŌËáÓëŌŌīžÔÚÅĻÁōËáŨöīßŧŊžÁĄĒÎüËŪžÁĖõžþÏÂÉúģÉŌŌËáŌŌõĨšÍËŪCH3COOHĢŦCH3CH2OH![]() CH3COOCH2CH3ĢŦH2OĢŧ
CH3COOCH2CH3ĢŦH2OĢŧ
īð°ļĢšCH3COOHĢŦCH3CH2OH![]() CH3COOCH2CH3ĢŦH2O
CH3COOCH2CH3ĢŦH2O
ĢĻ2ĢĐÅĻÁōËáūßÓÐÎüËŪÐÔĢŽÔÚŌŌËáÓëŌŌīž·ĒÉúõĨŧŊ·īÓĶĘąŨũīßŧŊžÁšÍÎüËŪžÁĢŧ
īð°ļĢšīßŧŊžÁĄĒÎüËŪžÁĢŧ
ĢĻ3ĢĐÉýļßÎÂķČŋÉŌÔžÓŋė·īÓĶËŲÂĘĢŽÍŽĘąŌŌīžĄĒŌŌËáŌŨŧÓ·ĒĢŽËųŌÔÎÂķČēŧÄÜđýļßĢŽ·ĀÖđ·īÓĶÎïΊĀīĩÞ°·īÓĶķøŧÓ·ĒËðʧĢŽÓĶÓÃÐĄŧðžÓČČĢŧšóļÄÓÃīóŧðžÓČČĩÄÄŋĩÄĘĮÕôģöÉúģÉĩÄŌŌËáŌŌõĨĢŽĘđŋÉÄæ·īÓĶÏōÓŌ―øÐÐĢŧ
īð°ļĢšžÓŋė·īÓĶËŲÂĘĢŽÍŽĘą·ĀÖđ·īÓĶÎïΊĀīĩÞ°·īÓĶķøŧÓ·ĒËðʧĢŧÕôģöÉúģÉĩÄŌŌËáŌŌõĨĢŽĘđŋÉÄæ·īÓĶÏōÓŌ―øÐÐĄĢ
ĢĻ4ĢĐŌŌËáŌŌõĨēŧČÜÓÚąĨšÍĖžËáÄÆČÜŌšĢŽķþÕßŧėšÏ·ÖēãĢŽŋÉŌÔÓ÷ÖŌš·Ļ·ÖĀëĢŽÖũŌŠŌĮÆũΊĢš·ÖŌšÂĐķ·Ģŧ
đĘīð°ļΊĢš·ÖŌšĢŧ·ÖŌšÂĐķ·Ģŧ

ĄūĖâÄŋĄŋ(1)ēÎŋžšÏģÉ·īÓĶCO(g)+2H2(g)![]() CH3OH(g)ĩÄÆ―šâģĢĘýĢŽŧØīðÏÂÁÐÎĘĖâĢš
CH3OH(g)ĩÄÆ―šâģĢĘýĢŽŧØīðÏÂÁÐÎĘĖâĢš
ÎÂķČ/Ąæ | 0 | 50 | 100 | 200 | 300 | 400 |
Æ―šâģĢĘý | 667 | 100 | 13 | 1.9ĄÁ10-2 | 2.4ĄÁ10-4 | 1ĄÁ10-5 |
ĒŲļ÷īÓĶÕý·īÓĶĘĮ___________ĢĻĖ·ÅČČĄąŧōĄ°ÎüČČĄąĢĐ·īÓĶĢŧ
ĒÚÔÚTĄæĘąĢŽ1LÃÜąÕČÝÆũÖÐĢŽÍķČë0.1molCOšÍ0.2molH2ĢŽīïĩ―Æ―šâĘąĢŽCOŨŠŧŊÂĘΊ50%ĢŽÔōT=__________ĄæĄĢ
(2)CH3OHŌēŋÉÓÉCO2šÍH2šÏģÉĄĢÔÚĖåŧýΊ1LĩÄÃÜąÕČÝÆũÖÐĢŽģäČëlmolCO2šÍ3molH2ĢŽŌŧķĻĖõžþÏ·īÓĶĢšCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ĶĪH=-49.0kJ/molĢŽēâĩÃCO2šÍCH3OH(g)ÅĻķČËæĘąžäąäŧŊČįÍžËųĘūĄĢ
CH3OH(g)+H2O(g) ĶĪH=-49.0kJ/molĢŽēâĩÃCO2šÍCH3OH(g)ÅĻķČËæĘąžäąäŧŊČįÍžËųĘūĄĢ
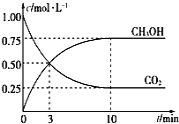
ĒŲļ÷īÓĶĩÄÆ―šâģĢĘýąíīïĘ―ÎŠK=________ĢŧīÓ·īÓĶŋŠĘžĩ―10minĢŽv(H2)=______molĄĪL-1ĄĪmin-1Ģŧ
ĒÚÏÂÁÐĮéŋöÄÜËĩÃũļ÷īÓĶŌŧķĻīïĩ―Æ―šâŨīĖŽĩÄĘĮ___________(ĖîŨÖÄļ)
A.v(CO2)ÏûšÄ=v(CH3OH)ÉúģÉ
B.ÆøĖåĩÄÃÜķČēŧÔŲËæĘąžäļÄąä
C.CO2šÍCH3OHĩÄÅĻķČÖŪąČēŧÔŲËæĘąžäļÄąä
D.ÆøĖåĩÄÆ―ūųÏāķÔ·ÖŨÓÖĘÁŋēŧÔŲËæĘąžäļÄąä
ĒÛΊÁËžÓŋėŧŊŅ§·īÓĶËŲÂĘĮŌĘđĖåÏĩÖÐÆøĖåĩÄÎïÖĘĩÄÁŋÔöīóĢŽÖŧļÄąäÏÂÁÐÄģŌŧĖõžþĢŽŋÉēÉČĄĩÄīëĘĐÓÐ___________ (ĖîŨÖÄļ)
A.ÉýļßÎÂķČ B.ËõÐĄČÝÆũĖåŧý C.ÔŲģäČëCO2ÆøĖå D.ĘđÓÚÏĘĘĩÄīßŧŊžÁ
ĒÜÏāÍŽÎÂķČÏÂĢŽÔÚÁíŌŧļöČÝŧýΊ1 LĩÄÃÜąÕČÝÆũÖÐģäČë2mol CH3OH(g)šÍ2molH2O(g)ĢŽīïĩ―Æ―šâĘąCO2ĩÄÅĻķČ____________(ĖĢūĄąĄĒĄ°ĢžĄąŧōĄ°=Ąą)0.25molĄĪL-1ĄĢ