��Ŀ����
15��������Ҫ�İ뵼����ϣ��������ִ����ӹ�ҵ�Ļ������ش��������⣺��1����̬Siԭ���У�����ռ�ݵ�����ܲ����M�����ܲ���е�ԭ�ӹ����Ϊ9��������Ϊ4��
��2������Ҫ�Թ����Ρ���������Ȼ��������ʽ�����ڵؿ��У�
��3�����ʹ��������ʯ�ṹ���Ƶľ��壬����ԭ����ԭ��֮���Թ��ۼ����ϣ��侧���й���8��ԭ�ӣ�����������λ�ù���3��ԭ�ӣ�
��4�����ʹ��ͨ�����飨SiH4���ֽⷴӦ���Ʊ�����ҵ�ϲ���Mg2Si��NH4CI��Һ�������з�Ӧ�Ƶ�SiH4���÷�Ӧ�Ļ�ѧ����ʽΪMg2Si+4NH4Cl=SiH4+4NH3+2MgCl2
��5��̼����йػ�ѧ������������ʾ����Ҫ�����ͽ��������й���ʵ��
| ��ѧ�� | C-C | C-H | C-O | Si-Si | Si-H | Si-O |
| ���ܣ�KJ/mol�� | 356 | 413 | 336 | 226 | 318 | 452 |
��SiH4���ȶ���С��CH4���������������ԭ����C-H���ļ��ܴ���C-O����C-H����C-O���ȶ�����Si-H���ļ���ȴԶС��Si-O��������Si-H�����ȶ����������γ��ȶ��Ը�ǿ��Si-O����
��6���ڹ������У�SiO44-�����壨����ͼa��ͨ�����ö��������ӿ��γɵ�״����״����״���Ǽ���״�Ĵ���ṹ��ʽ��ͼbΪһ�����������ṹ�Ķ�����������Siԭ�ӵ��ӻ���ʽΪsp3��Si��O��ԭ����֮��Ϊ1��3��ѧʽΪSiO32-��

���� ��1��ԭ���У���ԭ�Ӻ�ԽԶ�ĵ��Ӳ�������Խ�ߣ���ԭ���к���3��s�����6��p�����
��2������������Ԫ�أ�����Ȼ������Ҫ�Զ�����������δ��ڣ�
��3���ǽ���Ԫ��֮�����γɹ��ۼ������þ�̯�����㣻
��4��Mg2Si��NH4Cl��Һ�������з�Ӧ�Ƶ�SiH4��NH3��MgCl2��
��5���������е�C-C����C-H�����ڹ����е�Si-Si����Si-H���ļ��ܣ�
�ڼ���Խ�����ʾ�Խ�ȶ���C-H���ļ��ܴ���C-O������C-H����C-O���ȶ�����Si-H���ļ���ԶС��Si-O��������Si-H�����ȶ����������γ��ȶ��Ը�ǿ��Si-O����
��6������ͼ��b����һ���ṹ��Ԫ�к���1���衢3����ԭ�ӣ���ѧʽΪSiO32-��
��� �⣺��1��ԭ���У���ԭ�Ӻ�ԽԶ�ĵ��Ӳ�������Խ�ߣ�����Siԭ����M���Ӳ�������ߣ���ԭ���к���3��s�����6��p���������һ����9�������������Ϊ4��
�ʴ�Ϊ��M��9��4��
��2������������Ԫ�أ�����Ȼ���в����Ե��ʴ��ڣ���Ҫ�Զ�����������δ��ڣ��ʴ�Ϊ���������裻
��3���赥���й��֮���Թ��ۼ���ϣ��辧����ÿ����������1��Si����������1��Si���ھ����ڲ�����4��Siԭ�ӣ����þ�̯��֪�������ṩ�Ĺ�ԭ�Ӹ���=6��$\frac{1}{2}$=3��
�ʴ�Ϊ�����ۼ���3��
��4��Mg2Si��NH4Cl��Һ�������з�Ӧ�Ƶ�SiH4��NH3��MgCl2������ʽΪ��Mg2Si+4NH4Cl=SiH4+4NH3+2MgCl2���ʴ�Ϊ��Mg2Si+4NH4Cl=SiH4+4NH3+2MgCl2��
��5���������е�C-C����C-H�����ڹ����е�Si-Si����Si-H���ļ��ܣ����Թ�����Si-Si����Si-H���ļ������ѣ����³��������������ɣ�
�ʴ�Ϊ��C-C����C-H����ǿ�����γɵ������ȶ�����������Si-Si����Si-H���ļ��ܽϵͣ����ѣ����³��������������ɣ�
�ڼ���Խ�����ʾ�Խ�ȶ���C-H���ļ��ܴ���C-O������C-H����C-O���ȶ�����Si-H���ļ���ԶС��Si-O��������Si-H�����ȶ����������γ��ȶ��Ը�ǿ��Si-O����
�ʴ�Ϊ��C-H���ļ��ܴ���C-O����C-H����C-O���ȶ�����Si-H���ļ���ȴԶС��Si-O��������Si-H�����ȶ����������γ��ȶ��Ը�ǿ��Si-O����
��6������ͼ��b����һ���ṹ��Ԫ�к���1���衢2+2��$\frac{1}{2}$=3����ԭ�ӣ���ѧʽΪSiO32-������Siԭ�ӵ��ӻ���ʽ��sp3��
�ʴ�Ϊ��sp3��1��3��SiO32-��
���� �����Թ輰�仯����Ϊ���忼���˾����ļ��㡢��֪ʶ�㣬��Щ֪ʶ�㶼�ǿ����ȵ㣬�������ղ�������û���֪ʶ������⣬�״����ǣ�1����

| A�� | ��ˮ�ĵ����Ժܲ����ˮ���ǵ���� | |
| B�� | SO2ˮ��Һ�ĵ����Ժܺã�����SO2�ǵ���� | |
| C�� | ����״̬ʱͭ�ĵ����Ժܺã�����ͭ�ǵ���� | |
| D�� | �������ˮ�к�����״̬ʱ���ܵ��磬����������ǵ���� |
| A�� | �����Ӽ�ȡ�����ƹ��壬�и�ȡ�ú�ʣ����Ʊ���Ż�ԭ�Լ�ƿ�� | |
| B�� | ̽���¶ȶԻ�ѧ��Ӧ����Ӱ��ʱ���Ƚ����������������������Һ��Ϻ�����ˮԡ���� | |
| C�� | ����þ�治�����ʧ�����ϸɳ���𣬲�������ĭ����� | |
| D�� | �ڷ�Һ�����У�����Һ�ֲ�����������²�Һ��ų���Ȼ��ر����������ϲ�Һ����Ͽڵ��� |
| A�� | ����������ԭ��Ӧ����缫��Ӧʽ��Ni2++2e-�TNi | |
| B�� | �����۵ײ�����������ֻ��Pt | |
| C�� | ������Һ�д��ڵ�������ֻ��Fe2+��Zn2+ | |
| D�� | �������У����������ļ������������������Ӳ���� |
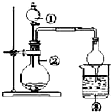 | ѡ�� | �� | �� | �� | ʵ����� |
| A | Ũ���� | MnO2 | NaBr��Һ | ��������Cl2��Br2 | |
| B | Ũ��ˮ | ��ʯ�� | AgNO3��Һ | AgOH�������� | |
| C | Ũ���� | Na2SO3 | FeCl3��Һ | SO2���л�ԭ�� | |
| D | ϡ���� | Na2CO3 | Na2SiO3 | �ǽ����ԣ�C��Si |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ������MnO2��������ŨHCl | B�� | ������ͭ��������Ũ������Һ | ||
| C�� | �����ĵ����������������ϳɰ� | D�� | �������������������� |
| A�� | �����22.4 L H2O�� 1 mol O2������������� | |
| B�� | 1 molˮ�к���2 mol���1 mol�� | |
| C�� | 1 mol��̬ˮ��1 molҺ̬ˮ�����ķ������� | |
| D�� | 3 mol O2 ��2 mol H2O����ԭ������� |
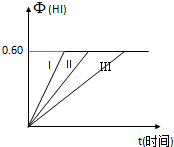 ��1molI2��g����2molH2����ij2L�ܱ������У���һ���¶��·�����Ӧ��
��1molI2��g����2molH2����ij2L�ܱ������У���һ���¶��·�����Ӧ�� ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й�����u���Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й�����u���Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺