��Ŀ����
��֪Ba(AlO2)2������ˮ����ͼ��ʾ������A12(SO4) 3��Һ����μ���Ba(OH)2��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ�������й�������ȷ����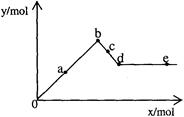
| A��a��bʱ���������ʵ���:A1(OH)3��BaSO4�� |
| B��c��dʱ��Һ�����ӵ����ʵ�����AlO2����Ba2+�� |
| C��a��dʱ���������ʵ�����BaSO4����С��A1(OH)3 |
| D��d��eʱ��Һ�����ӵ����ʵ�����Ba2+�����ܵ���OH�� |
B
���������������a��b������ӦAl2(SO4)3+3Ba(OH)2��2Al(OH)3��+3BaSO4�����ɷ���ʽ��֪�����������ʵ�����Al(OH)3��BaSO4����A����cd������Ӧ2Al(OH)3+Ba(OH)2=Ba(AlO2)2+4H2O��1molBa(AlO2)2�����2molAlO2����1molBa2������AlO2����Ba2���࣬��B��ȷ��ѡB��������1molAl2(SO4)3����Һ�к���2molAl3����3molSO42��������Һ����μ���Ba(OH)2��Һʱ�����ķ�ӦΪ��Al3��+3OH��=Al(OH)3����SO42��+Ba2��=BaSO4������2molAl3����ȫ����ʱ������Ba(OH)23mol����ʱ3molSO42��ȫ�����������ɳ���Ϊ2molAl(OH)3����3molBaSO4��5mol����ˣ���������Ӧ������BaSO4�����ʵ���ʼ�մ���Al(OH)3�������ʵ�������C����d��e���У�ij��ʱ�������Ba(OH)2�����ʵ�������Ba(AlO2)2�����ʵ���ʱ����Һ��Ba2����AlO2��������ȣ���D����
���㣺þ��������Ҫ�����

 ��У����ϵ�д�
��У����ϵ�д��������ӷ���ʽ����д��ȷ����
| A��SiO2��NaOH��Һ��Ӧ��SiO2+2Na++2OH��=Na2SiO3+ H2O |
| B��Ũ�ռ���Һ�м�����Ƭ��2Al + 2OH��+2 H2O��2AlO2��+3H2�� |
| C������ϡ���ᷴӦ��Fe + 2H+��Fe2+ + H 2�� |
| D��Cl2ͨ��ˮ�У�Cl2 + H2O�� 2H++Cl��+ClO�� |
��ˮ��Һ���ܴ��������һ��������
| A��Na����Al3����Cl����CO32�� | B��H����Na����Fe2����MnO4�� |
| C��K����Ca2����Cl����NO3�� | D��K����NH4����OH����SO42�� |
ijNa2CO3��NaAlO2�Ļ����Һ����μ���1 mol/L�����ᣬ�����Һ��CO32-��HCO3-��AlO2-��Al3�������ʵ��������������Һ������仯��ϵ��ͼ��ʾ��������˵����ȷ����
| A��ԭ�����Һ�е�CO32-��AlO2-�����ʵ���֮��Ϊ1��2 |
| B��V1��V2��l��5 |
| C��M��ʱ���ɵ�CO2Ϊ0��05 mol |
| D��a���߱�ʾ�����ӷ���ʽΪAlO2-��H����H2O==Al��OH��3�� |
������CO2�ֱ�ͨ�����и���Һ�У������������ܴ����������
| A��K+��AlO2-�� Cl-�� NO3- | B��Na+��CH3COO-��C6H5O-��HCO3- |
| C��Na+��ClO-��SO42-��Cl- | D��NH4+�� Al3+��SO42-��H+ |
ijNa2CO3��NaAlO2�Ļ����Һ����μ���1mol��L-1�����ᣬ�����Һ�е�CO32-��HCO3-��AlO2-��Al3+�����ʵ��������������Һ������仯��ϵ��ͼ��ʾ��������˵������ȷ����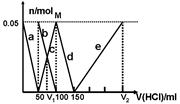
| A��M��ʱ���ɵ�CO2Ϊ0mol |
| B��ԭ�����Һ�е�CO32-��AlO2-�����ʵ���֮��Ϊ1��2 |
| C��V1��V2=1��4 |
| D��a���߱�ʾ�����ӷ���ʽΪ��AlO2- +H+ + H2O��Al(OH)3�� |
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ��
| A����������Һ�м��������ˮ Al3+��3OH-= Al(OH)3�� |
| B��̼������Һ�м������ʯ��ˮ Ca(OH)2��CO32-= CaCO3����2OH- |
| C���������������Һ��ͨ������ Cl2��2OH-= ClO-��Cl-��H2O |
| D��ϡ�����м������� 2Fe��6H+= 2Fe3+��3H2�� |
�����£����и���������ָ����Һ���ܴ����������
| A��pH��1����Һ�У�K+��Fe2+��MnO4-��SO42- |
| B��c(Fe3+)��0.1 mol��L-1����Һ�У�K+��ClO-��SO42-��SCN- |
| C��c(H+)/c (OH-)��1012����Һ�У�NH4+��Al3+��NO3-��Cl- |
| D��������Ӧ������������Һ�У�NH4+��K+��Cl-��SiO32- |
����˵����ȷ���ǣ� ��
| A��KClO3��SO3����ˮ���ܵ��磬��KClO3��SO3Ϊ����� |
| B����NaAlO2��Һ�еμ�NaHCO3��Һ���г������������� |
| C��25��ʱ���ô�����Һ�ζ���Ũ��NaOH��Һ��pH��7��V�����ᣩ> V��NaOH�� |
| D��AgCl��ת��ΪAgI������KSP(AgX)��c(Ag+)? c(X?)����KSP(AgI) > KSP(AgCl) |