��Ŀ����
6����NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ�������| A�� | һ�������£�1molN2��3mol H2��ַ�Ӧ���������е� N-H������ĿΪ6NA | |
| B�� | 23g NO2�� N2O4�Ļ������ԭ������Ϊ1.5NA | |
| C�� | 1L 0.1mol•L-1��Na2S��Һ��S2- �� HS- ������Ϊ0.1NA | |
| D�� | ��FeI2��Һ��ͨ������������������1molFe2+������ʱ���ܹ�ת�Ƶ��ӵ���ĿΪNA |
���� A���ϳɰ��ķ�ӦΪ���淴Ӧ�����ܽ��г��ף�
B��NO2�� N2O4�����ʽ��ΪNO2��
C������S2-�Ჿ��ˮ��ΪHS-��H2S�����������غ���������
D�����ݵ����Ӻ��������Ӷ��ܹ��������������������������������������ӷ�����
��� �⣺A���ϳɰ��ķ�ӦΪ���淴Ӧ�����ܽ��г��ף������ɵİ��������ʵ���С��1mol���ʺ��е�N-H�������ʵ���С��3NA������A����
B��NO2�� N2O4�����ʽ��ΪNO2����23g������к���NO2�����ʵ���n=$\frac{23g}{46g/mol}$=0.5mol���ʺ��е�ԭ������Ϊ1.5NA������B��ȷ��
C��S2-�������������Һ�лᲿ��ˮ��ΪHS-��H2S����SԪ������Һ�еĴ�����ʽ��S2-��HS-��H2S�����������غ��֪��n��S2-��+n��HS-��+n��H2S��=CV=0.1mol/L��1L=0.1mol����0.1NA������C����
D��FeI2��Һ�У������ӵĻ�ԭ�Դ����������ӵģ�ͨ����������������1molFe2+������ʱ����Һ�е������Ѿ���ȫ�����������ڲ�֪���⻯���������ʵ�����������ת�Ƶĵ���������D����
��ѡB��
���� ���⿼���˰���٤���������йؼ��㣬�������չ�ʽ��ʹ�ú����ʵĽṹ�ǽ���ؼ����ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ����Ҳ��Ϊ����ο����PM2.5������������ֱ�ӽ���֧���ܣ�����������������֧��������Ѫ�ܲ��ȷ���ļ��� | |
| B�� | �ߴ��ȵĹ赥�ʹ㷺�����������ά�����ά��ǿ��ᡰ��·�� | |
| C�� | ���ö�����̼��ԭ�Ϻϳɵľ�̼������ɽ��������������ϩ���� | |
| D�� | ������͵���Ҫ�ɷ������ô���ˮ����ȡ�ĵع�����״���Ӧ���ɵ�֬�������������ʯ�������б��ʲ�ͬ |
| A�� | ��ȩ�ͱ�ͪ | B�� | �Ҵ������� | ||
| C�� | ����ͻ����� | D�� | �ڼ����Ӻͱ��״� |
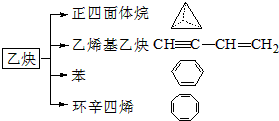
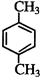
| A�� | ���ӻ������п��ܺ��зǼ��Լ� | |
| B�� | ԭ�Ӿ����з��Ӽ�������Խǿ���۵�Խ�� | |
| C�� | ԭ�Ӿ����п��ܺ��зǼ��Լ� | |
| D�� | �Ȼ��ƾ����ۻ�ʱ���Ӽ��������� |
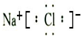 HCl
HCl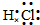
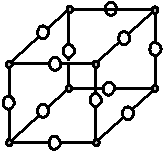 ����X��Y��Z��Wԭ�������������������Ԫ�أ�������XԪ�ص��ʵ��ܶ�����Ȼ������С��Y�Ƕ�����Ԫ����δ�ɶԵ�������ԭ������֮������ԭ�ӣ�ZԪ�ػ�̬ԭ�ӵĺ�������Ų�ʽ�У�s�Dz����������p�Dz����������ȣ���Y��Z���γɶ�����̬�����W�dz�������ɫ�ɱ�۽������ʣ������¿�����Y�����������ˮ�����У����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ1��
����X��Y��Z��Wԭ�������������������Ԫ�أ�������XԪ�ص��ʵ��ܶ�����Ȼ������С��Y�Ƕ�����Ԫ����δ�ɶԵ�������ԭ������֮������ԭ�ӣ�ZԪ�ػ�̬ԭ�ӵĺ�������Ų�ʽ�У�s�Dz����������p�Dz����������ȣ���Y��Z���γɶ�����̬�����W�dz�������ɫ�ɱ�۽������ʣ������¿�����Y�����������ˮ�����У����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ1��
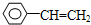
 ��
�� ��
��